Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells
- PMID: 32399219
- PMCID: PMC7203356
- DOI: 10.1007/s40201-019-00435-1
Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells
Abstract
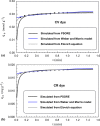
A new sorbent based on Sepia shells (cuttlefish bones) has been synthesized (SSBC) and tested for the sorption of cationic dye (crystal violet, CV) and an anionic dye (congo red, CR). SSBC was produced by reaction of sepia shells powder with urea in the presence of formaldehyde. In the first part of the work, the sorbent was characterized using scanning electron microscopy, energy dispersive X-ray analysis, Fourier-transform infra-red spectrometry and titration (for determining pHPZC). In a second step, sorption properties were tested on the two dyes through the study of pH effect, sorbent dosage, temperature and ionic strength; the sorption isotherms and uptake kinetics were analyzed at the optimum pH: Langmuir equation fits isotherm profiles while the kinetic profile can be described by the pseudo-second order rate equation. Maximum sorption capacities reach up to 0.536 mmol g-1 for CV and 0.359 mmol g-1 for CR, at pH 10.6 and 2.4, respectively. The comparison of sorption properties at different temperatures shows that the sorption is endothermic. Processing to the sorption under microwave irradiation (microwaved enforced sorption, MES) increases mass transfer and a contact time as low as 1 min is sufficient under optimized conditions (exposure time and power) reaching the equilibrium, while 2-3 h were necessary for "simple" sorption. Dye desorption was successfully tested using 0.5 M solutions of NaOH and HCl for the removal of CR and CV, respectively. The sorbent can be re-used for a minimum of four cycles of sorption/desorption. Finally, the sorbent was successfully tested on spiked tap water and real industrial wastewater.
Keywords: Industrial wastewater; Microwave enforced sorption; Sepia shells functionalization; Sorbent recycling; Sorption.
© Springer Nature Switzerland AG 2020.
Conflict of interest statement
Conflict of interestThe authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures







References
-
- Li Y, Bai P, Yan Y, Yan W, Shi W, Xu R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019;273:203–211. doi: 10.1016/j.micromeso.2018.07.010. - DOI
-
- Zhang J, Li F, Sun Q. Rapid and selective adsorption of cationic dyes by a unique metal-organic framework with decorated pore surface. Appl Surf Sci. 2018;440:1219–1226. doi: 10.1016/j.apsusc.2018.01.258. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

