Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples
- PMID: 32400364
- PMCID: PMC7219034
- DOI: 10.2807/1560-7917.ES.2020.25.18.2000603
Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples
Abstract
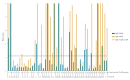
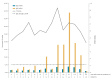
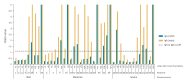
Antibody-screening methods to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) need to be validated. We evaluated SARS-CoV-2 IgG and IgA ELISAs in conjunction with the EUROLabworkstation (Euroimmun, Lübeck, Germany). Overall specificities were 91.9% and 73.0% for IgG and IgA ELISAs, respectively. Of 39 coronavirus disease patients, 13 were IgG and IgA positive and 11 IgA alone at sampling. IgGs and IgAs were respectively detected at a median of 12 and 11 days after symptom onset.
Keywords: COVID-19; IgA; IgG; SARS-CoV-2; commercial; serology.
Conflict of interest statement
Figures
References
-
- European Centre for Disease Prevention and Control (ECDC). Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK – ninth update, 23 April 2020. Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-...
-
- World Health Organization (WHO). Coronavirus disease (COVID-19) Pandemic. Geneva: WHO. [Accessed 28 Mar 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
-
- World Health Organization (WHO). Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 17 January 2020. Geneva: WHO; 17 Jan 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
