The role of natural products in revealing NRF2 function
- PMID: 32400766
- PMCID: PMC7316599
- DOI: 10.1039/c9np00061e
The role of natural products in revealing NRF2 function
Abstract
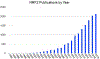
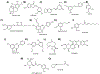
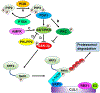
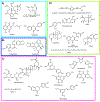
Covering: up to 2020The transcription factor NRF2 is one of the body's major defense mechanisms, driving transcription of >300 antioxidant response element (ARE)-regulated genes that are involved in many critical cellular processes including redox regulation, proteostasis, xenobiotic detoxification, and primary metabolism. The transcription factor NRF2 and natural products have an intimately entwined history, as the discovery of NRF2 and much of its rich biology were revealed using natural products both intentionally and unintentionally. In addition, in the last decade a more sinister aspect of NRF2 biology has been revealed. NRF2 is normally present at very low cellular levels and only activated when needed, however, it has been recently revealed that chronic, high levels of NRF2 can lead to diseases such as diabetes and cancer, and may play a role in other diseases. Again, this "dark side" of NRF2 was revealed and studied largely using a natural product, the quassinoid, brusatol. In the present review, we provide an overview of NRF2 structure and function to orient the general reader, we will discuss the history of NRF2 and NRF2-activating compounds and the biology these have revealed, and we will delve into the dark side of NRF2 and contemporary issues related to the dark side biology and the role of natural products in dissecting this biology.
Figures

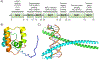

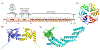
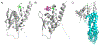

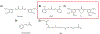
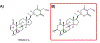
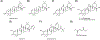
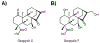
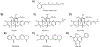
Similar articles
-
Molecular recognition between potential natural inhibitors of the Keap1-Nrf2 complex.Int J Biol Macromol. 2017 Dec;105(Pt 1):981-992. doi: 10.1016/j.ijbiomac.2017.07.117. Epub 2017 Jul 23. Int J Biol Macromol. 2017. PMID: 28746889
-
Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway.Chem Biodivers. 2019 Nov;16(11):e1900400. doi: 10.1002/cbdv.201900400. Epub 2019 Oct 10. Chem Biodivers. 2019. PMID: 31482617 Review.
-
The Keap1-Nrf2 protein-protein interaction: A suitable target for small molecules.Drug Discov Today Technol. 2017 Jun;24:11-17. doi: 10.1016/j.ddtec.2017.10.001. Epub 2017 Oct 31. Drug Discov Today Technol. 2017. PMID: 29233294 Review.
-
Emerging Substrate Proteins of Kelch-like ECH Associated Protein 1 (Keap1) and Potential Challenges for the Development of Small-Molecule Inhibitors of the Keap1-Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Protein-Protein Interaction.J Med Chem. 2020 Aug 13;63(15):7986-8002. doi: 10.1021/acs.jmedchem.9b01865. Epub 2020 Apr 8. J Med Chem. 2020. PMID: 32233486 Review.
-
Molecular mechanisms and systemic targeting of NRF2 dysregulation in cancer.Biochem Pharmacol. 2020 Jul;177:114002. doi: 10.1016/j.bcp.2020.114002. Epub 2020 Apr 28. Biochem Pharmacol. 2020. PMID: 32360363 Review.
Cited by
-
BACH1-Hemoxygenase-1 axis regulates cellular energetics and survival following sepsis.Free Radic Biol Med. 2022 Aug 1;188:134-145. doi: 10.1016/j.freeradbiomed.2022.06.005. Epub 2022 Jun 9. Free Radic Biol Med. 2022. PMID: 35691510 Free PMC article.
-
Short-chain fatty acids as modulators of redox signaling in health and disease.Redox Biol. 2021 Nov;47:102165. doi: 10.1016/j.redox.2021.102165. Epub 2021 Oct 14. Redox Biol. 2021. PMID: 34662811 Free PMC article. Review.
-
Stretch Causes cffDNA and HMGB1-Mediated Inflammation and Cellular Stress in Human Fetal Membranes.Int J Mol Sci. 2024 May 9;25(10):5161. doi: 10.3390/ijms25105161. Int J Mol Sci. 2024. PMID: 38791199 Free PMC article.
-
Sulforaphane Inhibits Foam Cell Formation and Atherosclerosis via Mechanisms Involving the Modulation of Macrophage Cholesterol Transport and the Related Phenotype.Nutrients. 2023 Apr 28;15(9):2117. doi: 10.3390/nu15092117. Nutrients. 2023. PMID: 37432260 Free PMC article.
-
In vitro and in silico hybrid approach to unveil triterpenoids from Helicteres hirsuta leaves as potential compounds for inhibiting Nrf2.RSC Adv. 2025 Jan 21;15(3):1915-1923. doi: 10.1039/d4ra07646j. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39839230 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

