A Fast and Clean BTK Inhibitor
- PMID: 32401033
- PMCID: PMC7304894
- DOI: 10.1021/acs.jmedchem.0c00597
A Fast and Clean BTK Inhibitor
Abstract
Bruton's tyrosine kinase (BTK) is a major drug target for B-cell related malignancies; however, existing BTK inhibitors approved for cancer treatment have significant off-targets that limit their use for autoimmune and inflammatory diseases. Remibrutinib (LOU064) is a novel covalent BTK inhibitor that binds an inactive BTK conformation, which affords it unprecedented selectivity. Its optimization led to rapid BTK engagement in vivo and fast clearance, further limiting systemic exposure. Remibrutinib is currently in phase 2 clinical trials for treatment of chronic urticaria and Sjoegren's syndrome.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
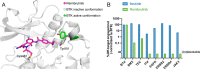
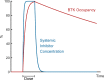
References
-
- Honigberg L. A.; Smith A. M.; Sirisawad M.; Verner E.; Loury D.; Chang B.; Li S.; Pan Z.; Thamm D. H.; Miller R. A.; Buggy J. J. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Blocks B-Cell Activation and Is Efficacious in Models of Autoimmune Disease and B-Cell Malignancy. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (29), 13075–13080. 10.1073/pnas.1004594107. - DOI - PMC - PubMed
-
- Angst D.; Gessier F.; Janser P.; Vulpetti A.; Wälchli R.; Beerli C.; Littlewood-Evans A.; Dawson J.; Nuesslein-Hildesheim B.; Wieczorek G.; Gutmann S.; Scheufler C.; Hinniger A.; Zimmerlin A.; Funhoff E. G.; Pulz R.; Cenni B. Discovery of LOU064 (Remibrutinib), a Potent and Highly Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J. Med. Chem. 2020, 10.1021/acs.jmedchem.9b01916. - DOI - PubMed
-
- Di Paolo J. A.; Huang T.; Balazs M.; Barbosa J.; Barck K. H.; Bravo B. J.; Carano R. A. D.; Darrow J.; Davies D. R.; DeForge L. E.; Diehl L.; Ferrando R.; Gallion S. L.; Giannetti A. M.; Gribling P.; Hurez V.; Hymowitz S. G.; Jones R.; Kropf J. E.; Lee W. P.; Maciejewski P. M.; Mitchell S. A.; Rong H.; Staker B. L.; Whitney J. A.; Yeh S.; Young W. B.; Yu C.; Zhang J.; Reif K.; Currie K. S. Specific Btk Inhibition Suppresses B Cell- and Myeloid Cell-Mediated Arthritis. Nat. Chem. Biol. 2011, 7 (1), 41–50. 10.1038/nchembio.481. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

