The serotonin reuptake blocker citalopram destabilizes fictive locomotor activity in salamander axial circuits through 5-HT1A receptors
- PMID: 32401145
- PMCID: PMC7311723
- DOI: 10.1152/jn.00179.2020
The serotonin reuptake blocker citalopram destabilizes fictive locomotor activity in salamander axial circuits through 5-HT1A receptors
Abstract
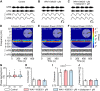
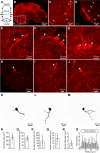
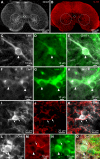
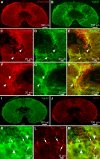
Serotoninergic (5-HT) neurons are powerful modulators of spinal locomotor circuits. Most studies on 5-HT modulation focused on the effect of exogenous 5-HT and these studies provided key information about the cellular mechanisms involved. Less is known about the effects of increased release of endogenous 5-HT with selective serotonin reuptake inhibitors. In mammals, such molecules were shown to destabilize the fictive locomotor output of spinal limb networks through 5-HT1A receptors. However, in tetrapods little is known about the effects of increased 5-HT release on the locomotor output of axial networks, which are coordinated with limb circuits during locomotion from basal vertebrates to mammals. Here, we examined the effect of citalopram on fictive locomotion generated in axial segments of isolated spinal cords in salamanders, a tetrapod where raphe 5-HT reticulospinal neurons and intraspinal 5-HT neurons are present as in other vertebrates. Using electrophysiological recordings of ventral roots, we show that fictive locomotion generated by bath-applied glutamatergic agonists is destabilized by citalopram. Citalopram-induced destabilization was prevented by a 5-HT1A receptor antagonist, whereas a 5-HT1A receptor agonist destabilized fictive locomotion. Using immunofluorescence experiments, we found 5-HT-positive fibers and varicosities in proximity with motoneurons and glutamatergic interneurons that are likely involved in rhythmogenesis. Our results show that increasing 5-HT release has a deleterious effect on axial locomotor activity through 5-HT1A receptors. This is consistent with studies in limb networks of turtle and mouse, suggesting that this part of the complex 5-HT modulation of spinal locomotor circuits is common to limb and axial networks in limbed vertebrates.NEW & NOTEWORTHY Little is known about the modulation exerted by endogenous serotonin on axial locomotor circuits in tetrapods. Using axial ventral root recordings in salamanders, we found that a serotonin reuptake blocker destabilized fictive locomotor activity through 5-HT1A receptors. Our anatomical results suggest that serotonin is released on motoneurons and glutamatergic interneurons possibly involved in rhythmogenesis. Our study suggests that common serotoninergic mechanisms modulate axial motor circuits in amphibians and limb motor circuits in reptiles and mammals.
Keywords: 5-HT1A; citalopram; locomotion; salamander; serotonin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Serotonergic Modulation of Locomotor Activity From Basal Vertebrates to Mammals.Front Neural Circuits. 2020 Nov 5;14:590299. doi: 10.3389/fncir.2020.590299. eCollection 2020. Front Neural Circuits. 2020. PMID: 33224027 Free PMC article. Review.
-
Divergent actions of serotonin receptor activation during fictive swimming in frog embryos.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004 May;190(5):391-402. doi: 10.1007/s00359-004-0504-9. Epub 2004 Feb 26. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004. PMID: 14991304
-
Mechanism of action of the bimodal antidepressant vilazodone: evidence for serotonin1A-receptor-mediated auto-augmentation of extracellular serotonin output.Psychopharmacology (Berl). 2014 Jun;231(12):2547-58. doi: 10.1007/s00213-013-3428-7. Epub 2014 Jan 14. Psychopharmacology (Berl). 2014. PMID: 24419272
-
Blockade of serotonin 5-HT1B and 5-HT2A receptors suppresses the induction of locomotor activity by 5-HT reuptake inhibitors, citalopram and fluvoxamine, in NMRI mice exposed to a novel environment: a comparison to other 5-HT receptor subtypes.Psychopharmacology (Berl). 2003 Aug;168(4):397-409. doi: 10.1007/s00213-003-1389-y. Epub 2003 Apr 30. Psychopharmacology (Berl). 2003. PMID: 12721776
-
Flexibility in the patterning and control of axial locomotor networks in lamprey.Integr Comp Biol. 2011 Dec;51(6):869-78. doi: 10.1093/icb/icr077. Epub 2011 Jul 9. Integr Comp Biol. 2011. PMID: 21743089 Free PMC article. Review.
Cited by
-
The role of torso stiffness and prediction in the biomechanics of anxiety: a narrative review.Front Sports Act Living. 2024 Nov 1;6:1487862. doi: 10.3389/fspor.2024.1487862. eCollection 2024. Front Sports Act Living. 2024. PMID: 39553377 Free PMC article. Review.
-
Investigation of central pattern generators in the spinal cord of chicken embryos.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Sep;210(5):801-814. doi: 10.1007/s00359-024-01694-6. Epub 2024 Mar 23. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 38521869 Free PMC article.
-
Serotonergic Modulation of Locomotor Activity From Basal Vertebrates to Mammals.Front Neural Circuits. 2020 Nov 5;14:590299. doi: 10.3389/fncir.2020.590299. eCollection 2020. Front Neural Circuits. 2020. PMID: 33224027 Free PMC article. Review.
-
From retina to motoneurons: A substrate for visuomotor transformation in salamanders.J Comp Neurol. 2022 Oct;530(14):2518-2536. doi: 10.1002/cne.25348. Epub 2022 Jun 3. J Comp Neurol. 2022. PMID: 35662021 Free PMC article.
References
-
- Adrio F, Anadón R, Rodríguez-Moldes I. Distribution of serotonin (5HT)-immunoreactive structures in the central nervous system of two chondrostean species (Acipenser baeri and Huso huso). J Comp Neurol 407: 333–348, 1999. doi:10.1002/(SICI)1096-9861(19990510)407:3<333:AID-CNE3>3.0.CO;2-R. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

