Experimentally Induced Endometritis Impairs the Developmental Capacity of Bovine Oocytes†
- PMID: 32401311
- PMCID: PMC7442778
- DOI: 10.1093/biolre/ioaa069
Experimentally Induced Endometritis Impairs the Developmental Capacity of Bovine Oocytes†
Erratum in
-
Corrigendum: Experimentally Induced Endometritis Impairs the Developmental Capacity of Bovine Oocytes†.Biol Reprod. 2020 Oct 5;103(4):907. doi: 10.1093/biolre/ioaa129. Biol Reprod. 2020. PMID: 32761062 Free PMC article. No abstract available.
Abstract
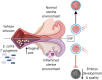
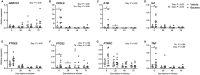
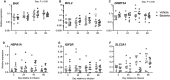
Uterine infection is associated with infertility in women and dairy cows, even after the resolution of infection. However, the mechanisms causing this persistent infertility are unclear. Here, we hypothesized that induced endometritis in non-lactating dairy cows would reduce the developmental competence of oocytes. Non-lactating Holstein cows received an intrauterine infusion of endometrial pathogenic bacteria (Escherichia coli and Trueperella pyogenes; n = 12) or vehicle control (n = 11) on day 2 of the estrous cycle. Bacterial infusion increased expression of endometrial inflammatory mediators, and a mucopurulent discharge in the vagina confirmed the establishment of endometritis. Oocytes were collected by transvaginal ultrasound-guided ovum pickup on days 2, 24, 45, and 66 following infusion and subjected to in vitro fertilization and embryo culture. Bacterial infusion resulted in fewer cleaved oocytes developing to morulae compared to vehicle-infused controls (30.7 versus 45.0%), with the greatest effect observed in oocytes collected on day 24. Development to morula was inversely correlated with endometrial expression of IL6 on day 6. The expression of genes associated with embryo quality did not differ significantly between morulae from bacteria-infused and control cows. Artificial insemination 130 days after intrauterine infusion resulted in normal, filamentous embryos that produced interferon tau 16 days after conception in both infusion groups. This model of experimentally induced uterine infection successfully resulted in endometritis and a reduction in the proportion of oocytes that developed to morulae following in vitro fertilization. In conclusion, endometritis reduced the capacity of oocytes to develop to morulae.
Keywords: embryo; endometritis; female infertility; inflammation; oocyte.
© The Author(s) 2020. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
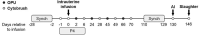
 ), endometrial cytobrush (
), endometrial cytobrush ( ) sampling, artificial insemination (AI), progesterone (P4) administration, and slaughter. Timeline is not drawn to scale.
) sampling, artificial insemination (AI), progesterone (P4) administration, and slaughter. Timeline is not drawn to scale.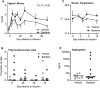
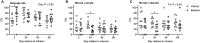



Similar articles
-
A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes.J Dairy Sci. 2019 Mar;102(3):2686-2697. doi: 10.3168/jds.2018-15595. Epub 2019 Jan 26. J Dairy Sci. 2019. PMID: 30692014 Free PMC article.
-
Dynamics of uterine infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in post-partum dairy cows and their association with clinical endometritis.Vet J. 2014 Dec;202(3):527-32. doi: 10.1016/j.tvjl.2014.08.023. Epub 2014 Aug 27. Vet J. 2014. PMID: 25439441
-
Effect of intrauterine infusion of ceftiofur on uterine health and fertility in dairy cows.J Dairy Sci. 2009 Apr;92(4):1532-42. doi: 10.3168/jds.2008-1615. J Dairy Sci. 2009. PMID: 19307634
-
Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows.J Dairy Sci. 2019 Dec;102(12):11786-11797. doi: 10.3168/jds.2019-17106. Epub 2019 Oct 3. J Dairy Sci. 2019. PMID: 31587913 Review.
-
Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle.Annu Rev Anim Biosci. 2019 Feb 15;7:361-384. doi: 10.1146/annurev-animal-020518-115227. Epub 2018 Oct 25. Annu Rev Anim Biosci. 2019. PMID: 30359085 Free PMC article. Review.
Cited by
-
Relationship between Oxidative Stress and Endometritis: Exploiting Knowledge Gained in Mares and Cows.Animals (Basel). 2022 Sep 13;12(18):2403. doi: 10.3390/ani12182403. Animals (Basel). 2022. PMID: 36139263 Free PMC article. Review.
-
The endometrial transcriptomic response to pregnancy is altered in cows after uterine infection.PLoS One. 2022 Mar 31;17(3):e0265062. doi: 10.1371/journal.pone.0265062. eCollection 2022. PLoS One. 2022. PMID: 35358206 Free PMC article.
-
Lipopolysaccharide alters CEBPβ signaling and reduces estradiol production in bovine granulosa cells.CABI Agric Biosci. 2022;3(1):66. doi: 10.1186/s43170-022-00133-3. Epub 2022 Oct 22. CABI Agric Biosci. 2022. PMID: 37576606 Free PMC article.
-
Impact of negative energy balance and postpartum diseases during the transition period on oocyte quality and embryonic development in dairy cows.Front Vet Sci. 2024 Jan 5;10:1328700. doi: 10.3389/fvets.2023.1328700. eCollection 2023. Front Vet Sci. 2024. PMID: 38249554 Free PMC article.
-
Induced endometrial inflammation compromises conceptus development in dairy cattle†.Biol Reprod. 2023 Oct 13;109(4):415-431. doi: 10.1093/biolre/ioad088. Biol Reprod. 2023. PMID: 37540198 Free PMC article.
References
-
- Centers for Disease Control and Prevention Sexually Transmitted Disease Surveillance 2017. Atlanta: U.S. Department of Health and Human Services; 2018. doi: 10.15620/cdc.59237. - DOI
-
- Deb K, Chaturvedi MM, Jaiswal YK. Comprehending the role of LPS in gram-negative bacterial vaginosis: Ogling into the causes of unfulfilled child-wish. Arch Gynecol Obstet 2004; 270:133–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

