Model of a Queuing Approach for Patient Accrual in Phase 1 Oncology Studies
- PMID: 32401317
- PMCID: PMC7221509
- DOI: 10.1001/jamanetworkopen.2020.4787
Model of a Queuing Approach for Patient Accrual in Phase 1 Oncology Studies
Abstract
Importance: Phase 1 cancer studies, which guide dose selection for subsequent studies, are almost 3 times more prevalent than phase 3 studies and have a median study duration considerably longer than 2 years, which constitutes a major component of drug development time.
Objective: To discern a method to reduce the duration of phase 1 studies in adult and pediatric cancer studies without violating risk limits by better accommodating the accrual and evaluation process (or queue).
Design: The process modeled, the phase 1 queue (IQ), includes patient interarrival time, screening, and dose-limiting toxicity evaluation. For this proof of principle, the rules of the 3 + 3 and rolling 6 phase 1 designs were modified to improve patient flow through the queue without exceeding the maximum risk permitted in the parent designs. The resulting designs, the IQ 3 + 3 and the IQ rolling 6, were each compared with their parent design by simulations in 12 different scenarios.
Main outcomes and measures: (1) The time from study opening to determination of the maximum tolerated dose (MTD), (2) the number of patients treated to determine the MTD, and (3) the association of the design with the dose selected as the MTD.
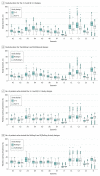
Results: Based on 800 simulations, for all 12 scenarios considered, the IQ 3 + 3 and the IQ rolling 6 designs were associated with reduced expected study durations compared with the parent design. The expected IQ 3 + 3 reduction ranged from 1.6 to 10.4 months (with 3.7 months for the standard scenario), and the expected reduction associated with IQ rolling 6 ranged from 0.4 to 10.5 months (with 3.4 months for the standard scenario). The increase in the mean number of patients treated in the IQ 3 + 3 compared with the 3 + 3 ranged from 0.6 to 3.2 patients. No increase in the number of patients was associated with the IQ rolling 6 compared with the rolling 6 design. The probability of selecting a dose level as the MTD changed by less than 3% for all dose levels and scenarios in both parent designs.
Conclusions and relevance: This study found that IQ designs were associated with reduced mean duration of phase 1 studies compared with their parent designs without changing the risk limits or MTD selection operating characteristics. These approaches have been successfully implemented in both hematology and solid tumor phase 1 studies.
Conflict of interest statement
Figures

References
-
- US Food and Drug Administration Step 3: clinical research. Published January 4, 2008. Accessed April 7, 2020. https://www.fda.gov/patients/drug-development-process/step-3-clinical-re...
-
- Alsumidaie M, Schiemann P Why are cancer clinical trials increasing in duration? Published 2015. Accessed April 24, 2020. http://www.appliedclinicaltrialsonline.com/why-are-cancer-clinical-trial...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

