Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
- PMID: 32401715
- PMCID: PMC7211500
- DOI: 10.1016/S0140-6736(20)31042-4
Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
Abstract
Background: Effective antiviral therapy is important for tackling the coronavirus disease 2019 (COVID-19) pandemic. We assessed the efficacy and safety of combined interferon beta-1b, lopinavir-ritonavir, and ribavirin for treating patients with COVID-19.
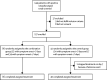
Methods: This was a multicentre, prospective, open-label, randomised, phase 2 trial in adults with COVID-19 who were admitted to six hospitals in Hong Kong. Patients were randomly assigned (2:1) to a 14-day combination of lopinavir 400 mg and ritonavir 100 mg every 12 h, ribavirin 400 mg every 12 h, and three doses of 8 million international units of interferon beta-1b on alternate days (combination group) or to 14 days of lopinavir 400 mg and ritonavir 100 mg every 12 h (control group). The primary endpoint was the time to providing a nasopharyngeal swab negative for severe acute respiratory syndrome coronavirus 2 RT-PCR, and was done in the intention-to-treat population. The study is registered with ClinicalTrials.gov, NCT04276688.
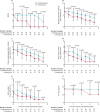
Findings: Between Feb 10 and March 20, 2020, 127 patients were recruited; 86 were randomly assigned to the combination group and 41 were assigned to the control group. The median number of days from symptom onset to start of study treatment was 5 days (IQR 3-7). The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab (7 days [IQR 5-11]) than the control group (12 days [8-15]; hazard ratio 4·37 [95% CI 1·86-10·24], p=0·0010). Adverse events included self-limited nausea and diarrhoea with no difference between the two groups. One patient in the control group discontinued lopinavir-ritonavir because of biochemical hepatitis. No patients died during the study.
Interpretation: Early triple antiviral therapy was safe and superior to lopinavir-ritonavir alone in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19. Future clinical study of a double antiviral therapy with interferon beta-1b as a backbone is warranted.
Funding: The Shaw-Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Interferon beta-1b for COVID-19.Lancet. 2020 May 30;395(10238):1670-1671. doi: 10.1016/S0140-6736(20)31101-6. Epub 2020 May 10. Lancet. 2020. PMID: 32401712 Free PMC article. No abstract available.
-
Early triple antiviral therapy for COVID-19.Lancet. 2020 Nov 7;396(10261):1487-1488. doi: 10.1016/S0140-6736(20)32274-1. Lancet. 2020. PMID: 33160566 Free PMC article. No abstract available.
-
Early triple antiviral therapy for COVID-19 - Authors' reply.Lancet. 2020 Nov 7;396(10261):1488. doi: 10.1016/S0140-6736(20)32268-6. Lancet. 2020. PMID: 33160568 Free PMC article. No abstract available.
References
-
- WHO Coronavirus disease 2019 (COVID-19) situation report–107. May 6, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Yao XH, Li TY, He ZC. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. (in Chinese). - PubMed
-
- Chan JF, Zhang AJ, Yuan S. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden syrian hamster model: implications for disease pathogenesis and tansmissibility. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa325. publised online March 26. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

