Vascularized human cortical organoids (vOrganoids) model cortical development in vivo
- PMID: 32401820
- PMCID: PMC7250475
- DOI: 10.1371/journal.pbio.3000705
Vascularized human cortical organoids (vOrganoids) model cortical development in vivo
Abstract
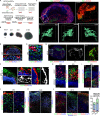
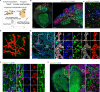
Modeling the processes of neuronal progenitor proliferation and differentiation to produce mature cortical neuron subtypes is essential for the study of human brain development and the search for potential cell therapies. We demonstrated a novel paradigm for the generation of vascularized organoids (vOrganoids) consisting of typical human cortical cell types and a vascular structure for over 200 days as a vascularized and functional brain organoid model. The observation of spontaneous excitatory postsynaptic currents (sEPSCs), spontaneous inhibitory postsynaptic currents (sIPSCs), and bidirectional electrical transmission indicated the presence of chemical and electrical synapses in vOrganoids. More importantly, single-cell RNA-sequencing analysis illustrated that vOrganoids exhibited robust neurogenesis and that cells of vOrganoids differentially expressed genes (DEGs) related to blood vessel morphogenesis. The transplantation of vOrganoids into the mouse S1 cortex resulted in the construction of functional human-mouse blood vessels in the grafts that promoted cell survival in the grafts. This vOrganoid culture method could not only serve as a model to study human cortical development and explore brain disease pathology but also provide potential prospects for new cell therapies for nervous system disorders and injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Approaches to vascularizing human brain organoids.PLoS Biol. 2023 May 8;21(5):e3002141. doi: 10.1371/journal.pbio.3002141. eCollection 2023 May. PLoS Biol. 2023. PMID: 37155714 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

