Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis
- PMID: 32402161
- PMCID: PMC7362958
- DOI: 10.1056/NEJMoa1915254
Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis
Abstract
Background: Bacterial vaginosis affects 15 to 50% of women of reproductive age, and recurrence is common after treatment with an antibiotic agent. The high incidence of recurrence suggests the need for new treatments to prevent recurrent bacterial vaginosis.
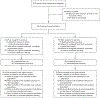
Methods: We conducted a randomized, double-blind, placebo-controlled, phase 2b trial to evaluate the ability of Lactobacillus crispatus CTV-05 (Lactin-V) to prevent the recurrence of bacterial vaginosis. Women 18 to 45 years of age who had received a diagnosis of bacterial vaginosis and who had completed a course of vaginal metronidazole gel as part of the eligibility requirements were randomly assigned, in a 2:1 ratio, to receive vaginally administered Lactin-V or placebo for 11 weeks; follow-up occurred through week 24. The primary outcome was the percentage of women who had a recurrence of bacterial vaginosis by week 12.
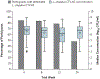
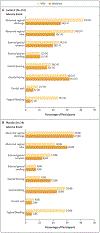
Results: A total of 228 women underwent randomization: 152 to the Lactin-V group and 76 to the placebo group; of these participants, 88% in the Lactin-V group and 84% in the placebo group could be evaluated for the primary outcome. In the intention-to-treat population, recurrence of bacterial vaginosis by week 12 occurred in 46 participants (30%) in the Lactin-V group and in 34 participants (45%) in the placebo group (risk ratio after multiple imputation for missing responses, 0.66; 95% confidence interval [CI], 0.44 to 0.87; P = 0.01). The risk ratio for recurrence by week 24 (also calculated with multiple imputation for missing responses) was 0.73 (95% CI, 0.54 to 0.92). At the 12-week visit, L. crispatus CTV-05 was detected in 79% of participants in the Lactin-V group. The percentage of participants who had at least one adverse event related to Lactin-V or placebo by week 24 did not differ significantly between the groups. The percentage of participants with local or systemic adverse events was similar in the two groups.
Conclusions: The use of Lactin-V after treatment with vaginal metronidazole resulted in a significantly lower incidence of recurrence of bacterial vaginosis than placebo at 12 weeks. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT02766023.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis.N Engl J Med. 2020 Aug 20;383(8):790-791. doi: 10.1056/NEJMc2021832. N Engl J Med. 2020. PMID: 32813958 No abstract available.
-
Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis.N Engl J Med. 2020 Aug 20;383(8):791. doi: 10.1056/NEJMc2021832. N Engl J Med. 2020. PMID: 32813959 No abstract available.
-
Excerpts from the World Medical Literature.J Obstet Gynaecol Can. 2020 Nov;42(11):1310-1313. doi: 10.1016/j.jogc.2020.09.006. J Obstet Gynaecol Can. 2020. PMID: 33189238 No abstract available.
References
-
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109: 114–20. - PubMed
-
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193: 1478–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
