Glucose Response by Stem Cell-Derived β Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis
- PMID: 32402282
- PMCID: PMC7433758
- DOI: 10.1016/j.celrep.2020.107623
Glucose Response by Stem Cell-Derived β Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis
Abstract
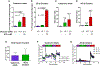
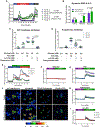
Stem cell-derived β (SC-β) cells could provide unlimited human β cells toward a curative diabetes treatment. Differentiation of SC-β cells yields transplantable islets that secrete insulin in response to glucose challenges. Following transplantation into mice, SC-β cell function is comparable to human islets, but the magnitude and consistency of response in vitro are less robust than observed in cadaveric islets. Here, we profile metabolism of SC-β cells and islets to quantify their capacity to sense glucose and identify reduced anaplerotic cycling in the mitochondria as the cause of reduced glucose-stimulated insulin secretion in SC-β cells. This activity can be rescued by challenging SC-β cells with intermediate metabolites from the TCA cycle and late but not early glycolysis, downstream of the enzymes glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase. Bypassing this metabolic bottleneck results in a robust, bi-phasic insulin release in vitro that is identical in magnitude to functionally mature human islets.
Keywords: GSIS; MIMOSA; differentiation; glucose-stimulated insulin secretion; metabolic profiling; stem cell metabolism; stem cell-derived β cell; β-cell metabolism.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests D.A.M. is the scientific founder of and an adviser for Semma Therapeutics and serves on the board of directors (BOD) of Blue Bird Bio.
Figures




References
-
- Bahl JJ, Matsuda M, DeFronzo RA, and Bressler R (1997). In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem. Pharmacol 53, 67–74. - PubMed
-
- Bonner-Weir S, Deery D, Leahy JL, and Weir GC (1989). Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 38, 49–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

