Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study)
- PMID: 32402554
- PMCID: PMC7483366
- DOI: 10.1016/j.ophtha.2020.03.021
Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study)
Abstract
Purpose: Assess follow-up treatment and clinical outcomes at 5 years in eyes initially treated with anti-VEGF therapy for center-involved diabetic macular edema (CI-DME) in a 2-year randomized clinical trial.
Design: Multicenter cohort study.
Participants: Participants with diabetic macular edema (DME) and visual acuity (VA) 20/32 to 20/320 enrolled in DRCR.net Protocol T with visits 5 years after randomization (3 years after Protocol T completion).
Methods: Participants were assigned randomly to aflibercept, bevacizumab, or ranibizumab with protocol-defined follow-up and re-treatment for 2 years. Thereafter, participants were managed at clinician discretion and recalled for a 5-year visit.
Main outcome measures: Anti-vascular endothelial growth factor (VEGF) treatment, VA letter score, and central subfield thickness (CST).
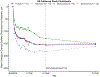
Results: Sixty-eight percent (317/463) of eligible participants completed the 5-year visit. Between years 2 and 5, 68% (217/317) of study eyes received at least 1 anti-VEGF treatment (median, 4; interquartile range [IQR], 0-12). At 5 years, mean VA improved from baseline by 7.4 letters (95% confidence interval [CI], 5.9-9.0) but decreased by 4.7 letters (95% CI, 3.3-6.0) between 2 and 5 years. When baseline VA was 20/50 to 20/320, mean 5-year VA was 11.9 letters (95% CI, 9.3-14.5) better than baseline but 4.8 letters (95% CI, 2.5-7.0) worse than 2 years. When baseline VA was 20/32 to 20/40, mean 5-year VA was 3.2 letters (95% CI, 1.4-5.0) better than baseline but 4.6 letters (95% CI, 3.1-6.1) worse than 2 years. Mean CST decreased from baseline to 5 years by 154 μm (95% CI, 142-166) and was stable between 2 and 5 years (-1 μm; 95% CI, -12 to 9).
Conclusions: Among the two-thirds of eligible Protocol T participants who completed a 5-year visit, mean VA improved from baseline to 5 years without protocol-defined treatment after follow-up ended at 2 years. Although mean retinal thickness was similar at 2 and 5 years, mean VA worsened during this period. Additional investigation into strategies to improve long-term outcomes in eyes with DME seems warranted to determine if VA can be better maintained with different management approaches.
Copyright © 2020 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. - PubMed
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(6):2247–2254. - PubMed
-
- Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema in the United States. Ophthalmol Retina. 2018;2(12):1179–1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

