Wide-Antimicrobial Spectrum of Picolinium Salts
- PMID: 32403238
- PMCID: PMC7248777
- DOI: 10.3390/molecules25092254
Wide-Antimicrobial Spectrum of Picolinium Salts
Abstract
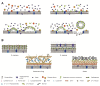
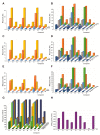
Nosocomial infections, which greatly increase morbidity among hospitalized patients, together with growing antibiotic resistance still encourage many researchers to search for novel antimicrobial compounds. Picolinium salts with different lengths of alkyl chains (C12, C14, C16) were prepared by Menshutkin-like reaction and evaluated with respect to their biological activity, i.e., lipophilicity and critical micellar concentration. Picolinium salts with C14 and C16 side chains achieved similar or even better results when in terms of antimicrobial efficacy than benzalkoniums; notably, their fungicidal efficiency was substantially more potent. The position of the methyl substituent on the aromatic ring does not seem to affect antimicrobial activity, in contrast to the effect of length of the N-alkyl chain. Concurrently, picolinium salts exhibited satisfactory low cytotoxicity against mammalian cells, i.e., lower than that of benzalkonium compounds, which are considered as safe.
Keywords: antimicrobial activity; critical micellar concentration; cytotoxicity; picolinium salts; quaternary ammonium compounds; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
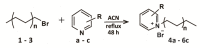


Similar articles
-
6-Hydroxyquinolinium salts differing in the length of alkyl side-chain: synthesis and antimicrobial activity.Bioorg Med Chem Lett. 2014 Nov 15;24(22):5238-41. doi: 10.1016/j.bmcl.2014.09.060. Epub 2014 Sep 28. Bioorg Med Chem Lett. 2014. PMID: 25442318
-
Synthesis and disinfection effect of the pyridine-4-aldoxime based salts.Molecules. 2015 Feb 24;20(3):3681-96. doi: 10.3390/molecules20033681. Molecules. 2015. PMID: 25719739 Free PMC article.
-
The wide-spectrum antimicrobial effect of novel N-alkyl monoquaternary ammonium salts and their mixtures; the QSAR study against bacteria.Eur J Med Chem. 2020 Nov 15;206:112584. doi: 10.1016/j.ejmech.2020.112584. Epub 2020 Jul 19. Eur J Med Chem. 2020. PMID: 32853858
-
Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions?ChemMedChem. 2012 Jan 2;7(1):22-31. doi: 10.1002/cmdc.201100404. Epub 2011 Nov 24. ChemMedChem. 2012. PMID: 22113995 Review.
-
Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts.Int J Mol Sci. 2015 Feb 6;16(2):3626-55. doi: 10.3390/ijms16023626. Int J Mol Sci. 2015. PMID: 25667977 Free PMC article. Review.
Cited by
-
Sustainable triazine-derived quaternary ammonium salts as antimicrobial agents.RSC Adv. 2021 Aug 19;11(45):28092-28096. doi: 10.1039/d1ra03455c. eCollection 2021 Aug 16. RSC Adv. 2021. PMID: 35480717 Free PMC article.
-
Styrylpyridinium Derivatives for Fluorescent Cell Imaging.Pharmaceuticals (Basel). 2023 Sep 4;16(9):1245. doi: 10.3390/ph16091245. Pharmaceuticals (Basel). 2023. PMID: 37765053 Free PMC article.
-
Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials.Int J Mol Sci. 2021 Jun 24;22(13):6793. doi: 10.3390/ijms22136793. Int J Mol Sci. 2021. PMID: 34202677 Free PMC article. Review.
-
Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative.Antibiotics (Basel). 2025 Mar 19;14(3):321. doi: 10.3390/antibiotics14030321. Antibiotics (Basel). 2025. PMID: 40149131 Free PMC article.
-
Efficacy of Novel Quaternary Ammonium and Phosphonium Salts Differing in Cation Type and Alkyl Chain Length against Antibiotic-Resistant Staphylococcus aureus.Int J Mol Sci. 2023 Dec 29;25(1):504. doi: 10.3390/ijms25010504. Int J Mol Sci. 2023. PMID: 38203676 Free PMC article.
References
-
- Kuca K., Kivala M., Dohnal V. A general method for the quaternization of N,N-dimethyl benzylamines with long chain n-alkylbromides. J. Appl. Biomed. 2004;2:195–198. doi: 10.32725/jab.2004.023. - DOI
-
- Augusta S., Gruber H.F., Streichsbier F. Synthesis and antibacterial activity of immobilized quaternary ammonium salts. J. Appl. Polym. Sci. 1994;53:1149–1163. doi: 10.1002/app.1994.070530903. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

