Wide-Antimicrobial Spectrum of Picolinium Salts
- PMID: 32403238
- PMCID: PMC7248777
- DOI: 10.3390/molecules25092254
Wide-Antimicrobial Spectrum of Picolinium Salts
Abstract
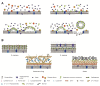
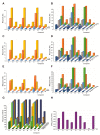
Nosocomial infections, which greatly increase morbidity among hospitalized patients, together with growing antibiotic resistance still encourage many researchers to search for novel antimicrobial compounds. Picolinium salts with different lengths of alkyl chains (C12, C14, C16) were prepared by Menshutkin-like reaction and evaluated with respect to their biological activity, i.e., lipophilicity and critical micellar concentration. Picolinium salts with C14 and C16 side chains achieved similar or even better results when in terms of antimicrobial efficacy than benzalkoniums; notably, their fungicidal efficiency was substantially more potent. The position of the methyl substituent on the aromatic ring does not seem to affect antimicrobial activity, in contrast to the effect of length of the N-alkyl chain. Concurrently, picolinium salts exhibited satisfactory low cytotoxicity against mammalian cells, i.e., lower than that of benzalkonium compounds, which are considered as safe.
Keywords: antimicrobial activity; critical micellar concentration; cytotoxicity; picolinium salts; quaternary ammonium compounds; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
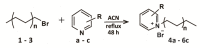


References
-
- Kuca K., Kivala M., Dohnal V. A general method for the quaternization of N,N-dimethyl benzylamines with long chain n-alkylbromides. J. Appl. Biomed. 2004;2:195–198. doi: 10.32725/jab.2004.023. - DOI
-
- Augusta S., Gruber H.F., Streichsbier F. Synthesis and antibacterial activity of immobilized quaternary ammonium salts. J. Appl. Polym. Sci. 1994;53:1149–1163. doi: 10.1002/app.1994.070530903. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

