The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury
- PMID: 32403251
- PMCID: PMC7278565
- DOI: 10.3390/antiox9050409
The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury
Abstract
In patients with abdominal region cancers, ionizing radiation (IR)-induced long-term liver injury is a major limiting factor in the use of radiotherapy. Previously, the major mitochondrial deacetylase, sirtuin 3 (SIRT3), has been implicated to play an important role in the development of acute liver injury after total body irradiation but no studies to date have examined the role of SIRT3 in liver's chronic response to radiation. In the current study, ten-month-old Sirt3-/- and Sirt3+/+ male mice received 24 Gy radiation targeted to liver. Six months after exposure, irradiated Sirt3-/- mice livers demonstrated histopathological elevations in inflammatory infiltration, the loss of mature bile ducts and higher DNA damage (TUNEL) as well as protein oxidation (3-nitrotyrosine). In addition, increased expression of inflammatory chemokines (IL-6, IL-1β, TGF-β) and fibrotic factors (Procollagen 1, α-SMA) were also measured in Sirt3-/- mice following 24 Gy IR. The alterations measured in enzymatic activities of catalase, glutathione peroxidase, and glutathione reductase in the livers of irradiated Sirt3-/- mice also implied that hydrogen peroxide and hydroperoxide sensitive signaling cascades in the absence of SIRT3 might contribute to the IR-induced long-term liver injury.
Keywords: hydroperoxide; inflammation; ionizing radiation; liver; sirtuin 3.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


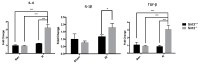






References
-
- Ben-Josef E., Normolle D., Ensminger W.D., Walker S., Tatro D., Ten Haken R.K., Knol J., Dawson L.A., Pan C., Lawrence T.S. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2005 doi: 10.1200/JCO.2005.01.5354. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

