Rheumatic Manifestations in Patients Treated with Immune Checkpoint Inhibitors
- PMID: 32403289
- PMCID: PMC7247001
- DOI: 10.3390/ijms21093389
Rheumatic Manifestations in Patients Treated with Immune Checkpoint Inhibitors
Abstract
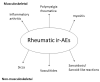
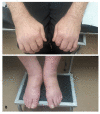
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that activate the immune system, aiming at enhancing antitumor immunity. Their clinical efficacy is well-documented, but the side effects associated with their use are still under investigation. These drugs cause several immune-related adverse events (ir-AEs), some of which stand within the field of rheumatology. Herein, we present a literature review performed in an effort to evaluate all publicly available clinical data regarding rheumatic manifestations associated with ICIs. The most common musculoskeletal ir-AEs are inflammatory arthritis, polymyalgia rheumatica and myositis. Non-musculoskeletal rheumatic manifestations are less frequent, with the most prominent being sicca, vasculitides and sarcoidosis. Cases of systemic lupus erythematosus or scleroderma are extremely rare. The majority of musculoskeletal ir-AEs are of mild/moderate severity and can be managed with steroids with no need for ICI discontinuation. In severe cases, more intense immunosuppressive therapy and permanent ICI discontinuation may be employed. Oncologists should periodically screen patients receiving ICIs for new-onset inflammatory musculoskeletal complaints and seek a rheumatology consultation in cases of persisting symptoms.
Keywords: arthritis; cancer immunotherapy; immune checkpoint inhibitors; musculoskeletal; myositis; polymyalgia rheumatica; rheumatic; scleroderma; sicca; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

