Regulation of Iron Homeostasis and Use in Chloroplasts
- PMID: 32403383
- PMCID: PMC7247011
- DOI: 10.3390/ijms21093395
Regulation of Iron Homeostasis and Use in Chloroplasts
Abstract
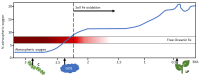
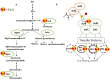
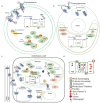
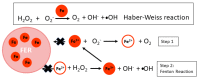
Iron (Fe) is essential for life because of its role in protein cofactors. Photosynthesis, in particular photosynthetic electron transport, has a very high demand for Fe cofactors. Fe is commonly limiting in the environment, and therefore photosynthetic organisms must acclimate to Fe availability and avoid stress associated with Fe deficiency. In plants, adjustment of metabolism, of Fe utilization, and gene expression, is especially important in the chloroplasts during Fe limitation. In this review, we discuss Fe use, Fe transport, and mechanisms of acclimation to Fe limitation in photosynthetic lineages with a focus on the photosynthetic electron transport chain. We compare Fe homeostasis in Cyanobacteria, the evolutionary ancestors of chloroplasts, with Fe homeostasis in green algae and in land plants in order to provide a deeper understanding of how chloroplasts and photosynthesis may cope with Fe limitation.
Keywords: Chlamydomonas; Cyanobacteria; Fe–S; chloroplast; green lineage; iron homeostasis; photosynthesis; plants.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Wacey D., Saunders M., Brasier M.D., Kilburn M.R. Earliest microbially mediated pyrite oxidation in similar to 3.4 billion-year-old sediments. Earth Planet. Sci. Lett. 2011;301:393–402. doi: 10.1016/j.epsl.2010.11.025. - DOI
-
- Fischer W., Hemp J., Johnson J. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016;44:647–683. doi: 10.1146/annurev-earth-060313-054810. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

