Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity
- PMID: 32404031
- PMCID: PMC7386868
- DOI: 10.1161/CIRCRESAHA.119.316463
Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity
Abstract
Rationale: Endothelial cells (ECs) are highly glycolytic and generate the majority of their energy via the breakdown of glucose to lactate. At the same time, a main role of ECs is to allow the transport of glucose to the surrounding tissues. GLUT1 (glucose transporter isoform 1/Slc2a1) is highly expressed in ECs of the central nervous system (CNS) and is often implicated in blood-brain barrier (BBB) dysfunction, but whether and how GLUT1 controls EC metabolism and function is poorly understood.
Objective: We evaluated the role of GLUT1 in endothelial metabolism and function during postnatal CNS development as well as at the adult BBB.
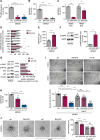
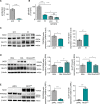
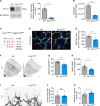
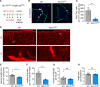
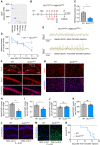
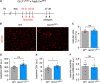
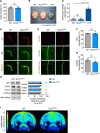
Methods and results: Inhibition of GLUT1 decreases EC glucose uptake and glycolysis, leading to energy depletion and the activation of the cellular energy sensor AMPK (AMP-activated protein kinase), and decreases EC proliferation without affecting migration. Deletion of GLUT1 from the developing postnatal retinal endothelium reduces retinal EC proliferation and lowers vascular outgrowth, without affecting the number of tip cells. In contrast, in the brain, we observed a lower number of tip cells in addition to reduced brain EC proliferation, indicating that within the CNS, organotypic differences in EC metabolism exist. Interestingly, when ECs become quiescent, endothelial glycolysis is repressed, and GLUT1 expression increases in a Notch-dependent fashion. GLUT1 deletion from quiescent adult ECs leads to severe seizures, accompanied by neuronal loss and CNS inflammation. Strikingly, this does not coincide with BBB leakiness, altered expression of genes crucial for BBB barrier functioning nor reduced vascular function. Instead, we found a selective activation of inflammatory and extracellular matrix related gene sets.
Conclusions: GLUT1 is the main glucose transporter in ECs and becomes uncoupled from glycolysis during quiescence in a Notch-dependent manner. It is crucial for developmental CNS angiogenesis and adult CNS homeostasis but does not affect BBB barrier function.
Keywords: blood-brain barrier; endothelium; extracellular matrix; glucose transport; glycolysis; homeostasis.
Figures

References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 20078464–478doi: 10.1038/nrm2183 - PubMed
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011146873–887doi: 10.1016/j.cell.2011.08.039 - PubMed
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 2007100158–173doi: 10.1161/01.RES.0000255691.76142.4a - PubMed
-
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013154651–663doi: 10.1016/j.cell.2013.06.037 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

