Effect of neoadjuvant radiotherapy on survival of non-metastatic pancreatic ductal adenocarcinoma: a SEER database analysis
- PMID: 32404114
- PMCID: PMC7222314
- DOI: 10.1186/s13014-020-01561-z
Effect of neoadjuvant radiotherapy on survival of non-metastatic pancreatic ductal adenocarcinoma: a SEER database analysis
Abstract
Background: Neoadjuvant radiotherapy has been shown to improve marginal negative resection and local control of Pancreatic Ductal Adenocarcinoma (PDAC). However, whether it improves overall survival (OS) in patients with non-metastatic PDAC remains controversial. Therefore, the purpose of this study was to analyze the benefits of only surgery, neoadjuvant radiotherapy, adjuvant radiotherapy, and surgery plus chemotherapy for OS in patients with non-metastatic PDAC.
Methods: PDAC diagnosed by surgical histopathology in the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2016 was selected. Kaplan-Meier analysis was used to compare the prognosis of patients with different treatments. Cox proportional risk model was used to analyze independent predictors of OS. Propensity score matching (PSM) was used to analyze the tumor prognosis of different treatment methods.
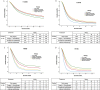
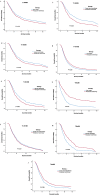
Results: Before PSM analysis, the OS of surgery plus chemotherapy (HRs = 0.896, 95%CIs, 0.827-0.970; P = 0.007) were significantly better than the other three treatments for stage T1-3N0M0 PDAC patients. For stage T1-3N + M0 patients, adjuvant radiotherapy (HRs = 0.613, 95% CIs, 0.579-0.649; P < 0.001) had significantly better OS than surgery plus chemotherapy and neoadjuvant radiotherapy. For stage T4N0M0 patients, neoadjuvant radiotherapy (HRs = 0.482, 95% CIs, 0.347-0.670; P < 0.001) had significantly better OS than surgery plus chemotherapy and adjuvant radiotherapy. For stage T4N + M0 patients, neoadjuvant radiotherapy (HRs = 0.338, 95% CIs, 0.215-0.532; P < 0.001) had significantly longer OS than adjuvant radiotherapy and surgery plus chemotherapy. Even after PSM, Chemotherapy plus surgery was still the best treatment for T1-3N0M0 patients. Postoperative adjuvant radiotherapy had the best prognosis among T1-3N + M0 patients, and neoadjuvant radiotherapy was the best treatment for T4 patients.
Conclusions: For patients with non-metastatic PDAC, neoadjuvant radiotherapy, adjuvant radiotherapy and surgery plus chemotherapy were superior to only surgery in OS. For patients with stage T4 non-metastatic PDAC, neoadjuvant radiotherapy had the potential to be strongly recommended over adjuvant radiotherapy and surgery plus chemotherapy. However, neoadjuvant radiotherapy failed to benefit the survival of T1-3N0M0 stage patients, and surgery plus chemotherapy was preferred. For T1-3N + M0, neoadjuvant radiotherapy had no obvious advantage over adjuvant radiotherapy or surgery plus chemotherapy in OS, and adjuvant radiotherapy was more recommended.
Keywords: Neoadjuvant radiotherapy; Overall survival; Pancreatic ductal adenocarcinoma; Propensity score matching; SEER.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The survival effect of neoadjuvant therapy and neoadjuvant plus adjuvant therapy on pancreatic ductal adenocarcinoma patients with different TNM stages: a propensity score matching analysis based on the SEER database.Expert Rev Anticancer Ther. 2024 Jun;24(6):467-476. doi: 10.1080/14737140.2024.2347513. Epub 2024 Apr 29. Expert Rev Anticancer Ther. 2024. PMID: 38656796
-
Survival Outcomes in Nonmetastatic pT4 Pancreatic Ductal Adenocarcinoma: A SEER Database Analysis Comparing Neoadjuvant Therapy and Upfront Surgery with Propensity Score Matching.Asian Pac J Cancer Prev. 2025 Mar 1;26(3):847-859. doi: 10.31557/APJCP.2025.26.3.847. Asian Pac J Cancer Prev. 2025. PMID: 40156401 Free PMC article.
-
Neoadjuvant radiotherapy improves overall survival for T3/4N+M0 rectal cancer patients: a population-based study of 20300 patients.Radiat Oncol. 2020 Feb 27;15(1):49. doi: 10.1186/s13014-020-01497-4. Radiat Oncol. 2020. PMID: 32103755 Free PMC article.
-
A single center experience in resectable pancreatic ductal adenocarcinoma : the limitations of the surgery-first approach. Critical review of the literature and proposals for practice update.Acta Gastroenterol Belg. 2017 Oct-Dec;80(4):451-461. Acta Gastroenterol Belg. 2017. Retraction in: Acta Gastroenterol Belg. 2018 Apr-Jun;81(2):358. PMID: 29560639 Retracted. Review.
-
The role of radiotherapy in multimodal treatment of pancreatic carcinoma.Radiat Oncol. 2010 Jul 8;5:64. doi: 10.1186/1748-717X-5-64. Radiat Oncol. 2010. PMID: 20615227 Free PMC article. Review.
Cited by
-
Photodynamic Stromal Depletion in Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2023 Aug 16;15(16):4135. doi: 10.3390/cancers15164135. Cancers (Basel). 2023. PMID: 37627163 Free PMC article. Review.
-
PI3Kγ inhibition combined with DNA vaccination unleashes a B-cell-dependent antitumor immunity that hampers pancreatic cancer.J Exp Clin Cancer Res. 2024 Jun 1;43(1):157. doi: 10.1186/s13046-024-03080-1. J Exp Clin Cancer Res. 2024. PMID: 38824552 Free PMC article.
-
The choice of adjuvant radiotherapy in pancreatic cancer patients after up-front radical surgery.PLoS One. 2025 Jan 24;20(1):e0317995. doi: 10.1371/journal.pone.0317995. eCollection 2025. PLoS One. 2025. PMID: 39854493 Free PMC article.
-
The survival impact of palliative radiotherapy on synchronous metastatic pancreatic ductal adenocarcinoma: metastatic site can serve for radiotherapy-decision.J Cancer. 2022 Jan 1;13(2):385-392. doi: 10.7150/jca.64800. eCollection 2022. J Cancer. 2022. PMID: 35069888 Free PMC article.
-
Efficacy of Neoadjuvant Radiotherapy After Chemotherapy and the Optimal Interval from Radiotherapy to Surgery for Borderline Resectable and Resectable Pancreatic Cancer.Ann Surg Oncol. 2025 Apr;32(4):2819-2829. doi: 10.1245/s10434-024-16743-2. Epub 2025 Jan 14. Ann Surg Oncol. 2025. PMID: 39808212 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

