Insecticide susceptibility of the sand fly leishmaniasis vector Phlebotomus argentipes in Sri Lanka
- PMID: 32404115
- PMCID: PMC7218544
- DOI: 10.1186/s13071-020-04117-y
Insecticide susceptibility of the sand fly leishmaniasis vector Phlebotomus argentipes in Sri Lanka
Abstract
Background: Leishmania donovani-induced and sand fly-transmitted leishmaniasis is a growing health problem in Sri Lanka. Limited knowledge on biological and behavioral characteristics of probable vector Phlebotomus argentipes hinders disease control. Here, insecticide susceptibility patterns of P. argentipes were investigated with exploration of probable underlying resistance mechanisms.
Methods: Adult sand flies were collected using standard cattle baited net traps and CDC light traps from selected sites in four districts. Adult F1 progeny of P. argentipes were exposed to different concentrations of DDT, malathion, deltamethrin and propoxur using WHO susceptibility bioassay kits. Post-1-h knockdown and post-24-h mortality were recorded and analyzed. Metabolic enzyme activity and the sensitivity of the acetylcholinesterase target-site were determined by biochemical assays using wild-caught flies. Extracted fly DNA samples were tested for the presence of knockdown-resistance (kdr) type mutations.
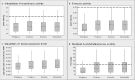
Results: The LC100 values for DDT, malathion, propoxur and deltamethrin were 0.8-1.5%, 0.9-2.0%, 0.017-0.03% and 0.007% respectively. Insecticide-susceptibility levels were higher than the discriminating dosages established for Aedes mosquitoes, except for malathion. The lowest susceptibility levels (except for deltamethrin) were detected in the Mamadala population, whereas the highest levels were detected in the Mirigama population. The percentage of knocked-down sand flies was < 75% at any tested concentration, including those, which exhibited 100% mortality after 24 h. Elevated activity levels of glutathione S-transferase (3%, 7%, 12.5% and 14%) and esterase (2%, 5%, 5.5% and 6.5%) were detected in flies that originated from Mirigama, Pannala, Thalawa and Mamadala respectively, while monooxygenase quantities remained below the cut-off level. Ten to 34.5% of flies were heterozygous for acetylcholinesterases target-site insensitivity, associated with organophosphate and carbamate resistance. Pyrethroid-resistance-associated L1014F kdr-type mutation in the voltage gated sodium channel gene was detected in 30/53 flies.
Conclusions: Populations of P. argentipes in Sri Lanka are largely susceptible to common insecticides, except for malathion (used extensively in the past for malaria control). Their insecticide susceptibility appears negatively associated with past malaria endemicity of the study sites, with signs of early insecticide tolerance. Presence of insecticide target site insensitivity in a notable proportion of flies and enhanced insecticide metabolizing enzyme activities imply potential future challenges for leishmaniasis control, with a call for urgent proactive measures for its containment.
Keywords: Bioassay; Biochemical analysis; Genetic mutation; Insecticide resistance; Insects; Sand fly; VGSC gene; Vector control; kdr mutation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- WHO. Leishmaniasis. 2019. Geneva: World Health Organization; 2019. https://www.who.int/gho/neglected_diseases/leishmaniasis/en/. Accessed 30 Aug 2019.
-
- Weekly Epidemiological reports: epidemiology unit. http://www.epid.gov.lk/. Accessed 30 Aug 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

