Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy
- PMID: 32404196
- PMCID: PMC7218526
- DOI: 10.1186/s13045-020-00885-3
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy
Abstract
Proteolysis-targeting chimera (PROTAC) has been developed to be a useful technology for targeted protein degradation. A bifunctional PROTAC molecule consists of a ligand (mostly small-molecule inhibitor) of the protein of interest (POI) and a covalently linked ligand of an E3 ubiquitin ligase (E3). Upon binding to the POI, the PROTAC can recruit E3 for POI ubiquitination, which is subjected to proteasome-mediated degradation. PROTAC complements nucleic acid-based gene knockdown/out technologies for targeted protein reduction and could mimic pharmacological protein inhibition. To date, PROTACs targeting ~ 50 proteins, many of which are clinically validated drug targets, have been successfully developed with several in clinical trials for cancer therapy. This article reviews PROTAC-mediated degradation of critical oncoproteins in cancer, particularly those in hematological malignancies. Chemical structures, cellular and in vivo activities, pharmacokinetics, and pharmacodynamics of these PROTACs are summarized. In addition, potential advantages, challenges, and perspectives of PROTAC technology in cancer therapy are discussed.
Keywords: Cancer therapy; Hematological malignancies; PROTAC; Targeted protein degradation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




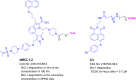
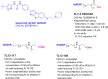
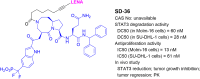

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

