Medication persistence on biological therapies prescribed for the treatment of chronic inflammatory arthropathies: a real-world data study
- PMID: 32404382
- PMCID: PMC8640415
- DOI: 10.1136/ejhpharm-2019-002133
Medication persistence on biological therapies prescribed for the treatment of chronic inflammatory arthropathies: a real-world data study
Abstract
Objectives: Medication persistence, defined as the duration of time from its initiation to its discontinuation, is a surrogate for treatment effectiveness. The aim of the study was to evaluate persistence and causes of biological therapy (BT) suspension in patients with chronic inflammatory arthropathies: rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis.
Methods: Single institution, descriptive, retrospective cohort study. Adult patients with chronic inflammatory arthropathies on BT between January 2009 and December 2016 were included. Persistence to BT was compared considering the type of pathology and treatment. The Kaplan-Meier test was used to analyse medication persistanence and factors associated with it. An analysis of reasons for therapy discontinuation was performed.
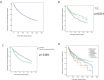
Results: Three hundred and sixty-two patients were included in the study, which comprised 478 BT lines. For all patients, the 12-month persistence rate was 71.3% (341 out of 478). At the end of the study, 45.2% of the patients continued on their initial BT. Median treatment persistence was 1489 days (CI 95% 1195 to 1783). Longer BT persistence was associated with naïve BT patients: 1945 days (95% CI 1523 to 2367; P<0.001) and ankylosing spondylitis diagnosis: 2402 days (95% CI 1604 to 3200; P=0.014). The most frequent causes of treatment discontinuation were therapeutic failure (47.6%) and adverse drug events (28.2%).
Conclusions: We found good long-term persistence in patients with chronic inflammatory arthropathies treated with BT. Patients with rheumatoid arthritis had significantly shorter persistence compared with those with ankylosing spondylitis and psoriatic arthritis. Naïve BT was associated with longer persistence. Therapeutic failure was the main cause of BT withdrawal.
Keywords: clinical pharmacy; health economics; public health; rheumatology; side effects of drugs.
© European Association of Hospital Pharmacists 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
