Biomarkers and Disease Severity in Children With Community-Acquired Pneumonia
- PMID: 32404432
- PMCID: PMC7263054
- DOI: 10.1542/peds.2019-3728
Biomarkers and Disease Severity in Children With Community-Acquired Pneumonia
Erratum in
-
Biomarkers and Disease Severity in Children With Community-Acquired Pneumonia.Pediatrics. 2020 Sep;146(3):e2020011452. doi: 10.1542/peds.2020-011452. Pediatrics. 2020. PMID: 32868465 Free PMC article. No abstract available.
Abstract
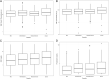
Background: Host biomarkers predict disease severity in adults with community-acquired pneumonia (CAP). We evaluated the association of the white blood cell (WBC) count, absolute neutrophil count (ANC), C-reactive protein (CRP), and procalcitonin with the development of severe outcomes in children with CAP.
Methods: We performed a prospective cohort study of children 3 months to 18 years of age with CAP in the emergency department. The primary outcome was disease severity: mild (discharged from the hospital), mild-moderate (hospitalized but not moderate-severe or severe), moderate-severe (eg, hospitalized with receipt of intravenous fluids, supplemental oxygen, complicated pneumonia), and severe (eg, intensive care, vasoactive infusions, chest drainage, severe sepsis). Outcomes were examined within the cohort with suspected CAP and in a subset with radiographic CAP.
Results: Of 477 children, there were no statistical differences in the median WBC count, ANC, CRP, or procalcitonin across severity categories. No biomarker had adequate discriminatory ability between severe and nonsevere disease (area under the curve [AUC]: 0.53-0.6 for suspected CAP and 0.59-0.64 for radiographic CAP). In analyses adjusted for age, antibiotic use, fever duration, and viral pathogen detection, CRP was associated with moderate-severe disease (odds ratio 1.12; 95% confidence interval, 1.0-1.25). CRP and procalcitonin revealed good discrimination of children with empyema requiring chest drainage (AUC: 0.83) and sepsis with vasoactive infusions (CRP AUC: 0.74; procalcitonin AUC: 0.78), although prevalence of these outcomes was low.
Conclusions: WBC count, ANC, CRP, and procalcitonin are generally not useful to discriminate nonsevere from severe disease in children with CAP, although CRP and procalcitonin may have some utility in predicting the most severe outcomes.
Copyright © 2020 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 - PubMed
-
- Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237–244 - PubMed
-
- Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192–1195 - PubMed
-
- Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr.. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–1525 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

