A Novel Negative Pressure-Flow Waveform to Ventilate Lungs for Normothermic Ex Vivo Lung Perfusion
- PMID: 32404613
- PMCID: PMC9218878
- DOI: 10.1097/MAT.0000000000001168
A Novel Negative Pressure-Flow Waveform to Ventilate Lungs for Normothermic Ex Vivo Lung Perfusion
Abstract
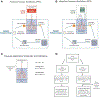
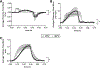
Ex vivo lung perfusion (EVLP) is increasingly used to treat and assess lungs before transplant. Minimizing ventilator induced lung injury (VILI) during EVLP is an important clinical need, and negative pressure ventilation (NPV) may reduce VILI compared with conventional positive pressure ventilation (PPV). However, it is not clear if NPV is intrinsically lung protective or if differences in respiratory pressure-flow waveforms are responsible for reduced VILI during NPV. In this study, we quantified lung injury using novel pressure-flow waveforms during normothermic EVLP. Rat lungs were ventilated-perfused ex vivo for 2 hours using tidal volume, positive end-expiratory pressure (PEEP), and respiratory rate matched PPV or NPV protocols. Airway pressures and flow rates were measured in real time and lungs were assessed for changes in compliance, pulmonary vascular resistance, oxygenation, edema, and cytokine secretion. Negative pressure ventilation lungs demonstrated reduced proinflammatory cytokine secretion, reduced weight gain, and reduced pulmonary vascular resistance (p < 0.05). Compliance was higher in NPV lungs (p < 0.05), and there was no difference in oxygenation between the two groups. Respiratory pressure-flow waveforms during NPV and PPV were significantly different (p < 0.05), especially during the inspiratory phase, where the NPV group exhibited rapid time-dependent changes in pressure and airflow whereas the PPV group exhibited slower changes in airflow/pressures. Lungs ventilated with PPV also had a greater transpulmonary pressure (p < 0.05). Greater improvement in lung function during NPV EVLP may be caused by favorable airflow patterns and/or pressure dynamics, which may better mimic human respiratory patterns.
Copyright © 2020 by the ASAIO.
Conflict of interest statement
Disclosure: The authors have no conflicts of interest to report.
Figures




References
-
- Rana A, Gruessner A, Agopian VG, et al.: Survival benefit of solidorgan transplant in the United States. JAMA Surg 150: 252–259, 2015. - PubMed
-
- Vandervest KM, Zamora MR: Recipient risk factors and lung transplant outcomes. Curr Opin Organ Transplant 18: 531–536, 2013. - PubMed
-
- Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO: Organ donation and utilization in the United States, 1999–2008. Am J Transplant 10(4 Pt 2): 973–986, 2010. - PubMed
-
- Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS: Organ donation and utilization in the United States, 1996–2005. Am J Transplant 7(5 Pt 2): 1327–1338, 2007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

