Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients
- PMID: 32404734
- PMCID: PMC7331824
- DOI: 10.1097/CMR.0000000000000669
Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients
Abstract
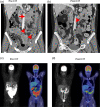
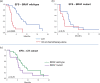
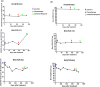
Management of PD-1 blockade resistance in metastatic melanoma (MM) remains challenging. Immunotherapy or chemotherapy alone provides limited benefit in this setting. Chemo-immunotherapy (CIT) has demonstrated favorable efficacy and safety profiles in lung cancer. Our pre-clinical study showed that in MM patients who have failed PD-1 blockade, the addition of chemotherapy increases CX3CR1+ therapy-responsive CD8+ T-cells with enhanced anti-tumor activity, resulting in improved clinical response. Here, we examined the clinical outcomes of CIT in MM patients after PD-1 blockade failure and the treatment-related changes in CX3CR1+ therapy-responsive CD8+ T-cells. We reviewed MM patients seen between January 2012 and June 2018 who failed anti-PD-1-based therapy and received subsequent CIT, immune checkpoint inhibitors (ICI) or chemotherapy alone. Overall survival (OS), objective response rate (ORR), event-free survival (EFS), and toxicities were assessed. Among 60 patients, 33 received CIT upon disease progression on PD-1 blockade. At a median follow-up of 3.9 years, the CIT group had a median OS of 3.5 years [95% confidence interval (CI) 1.7-NR] vs. 1.8 years (95% CI 0.9-2; P = 0.002) for those who received subsequent ICI (n = 9) or chemotherapy alone (n = 18), with ORR of 59% vs. 15% (P = 0.0003), respectively. The median EFS was 7.6 months (95% CI 6-10) following CIT vs. 3.4 months (95% CI 2.8-4.1; P = 0.0005) following ICI or chemotherapy alone. Therapy-responsive CX3CR1+CD8+ T-cells showed dynamic increase with successful CIT. CIT showed favorable clinical outcomes and acceptable safety profile in PD-1 blockade-resistant patients. CX3CR1+CD8+ therapy-responsive T-cells can be potentially used for monitoring disease response to CIT.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE, 3rd, et al. Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016; 14:450–473 - PubMed
-
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015; 28:690–714 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

