Composition-dependent thermodynamics of intracellular phase separation
- PMID: 32405004
- PMCID: PMC7733533
- DOI: 10.1038/s41586-020-2256-2
Composition-dependent thermodynamics of intracellular phase separation
Abstract
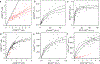
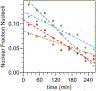
Intracellular bodies such as nucleoli, Cajal bodies and various signalling assemblies represent membraneless organelles, or condensates, that form via liquid-liquid phase separation (LLPS)1,2. Biomolecular interactions-particularly homotypic interactions mediated by self-associating intrinsically disordered protein regions-are thought to underlie the thermodynamic driving forces for LLPS, forming condensates that can facilitate the assembly and processing of biochemically active complexes, such as ribosomal subunits within the nucleolus. Simplified model systems3-6 have led to the concept that a single fixed saturation concentration is a defining feature of endogenous LLPS7-9, and has been suggested as a mechanism for intracellular concentration buffering2,7,8,10. However, the assumption of a fixed saturation concentration remains largely untested within living cells, in which the richly multicomponent nature of condensates could complicate this simple picture. Here we show that heterotypic multicomponent interactions dominate endogenous LLPS, and give rise to nucleoli and other condensates that do not exhibit a fixed saturation concentration. As the concentration of individual components is varied, their partition coefficients change in a manner that can be used to determine the thermodynamic free energies that underlie LLPS. We find that heterotypic interactions among protein and RNA components stabilize various archetypal intracellular condensates-including the nucleolus, Cajal bodies, stress granules and P-bodies-implying that the composition of condensates is finely tuned by the thermodynamics of the underlying biomolecular interaction network. In the context of RNA-processing condensates such as the nucleolus, this manifests in the selective exclusion of fully assembled ribonucleoprotein complexes, providing a thermodynamic basis for vectorial ribosomal RNA flux out of the nucleolus. This methodology is conceptually straightforward and readily implemented, and can be broadly used to extract thermodynamic parameters from microscopy images. These approaches pave the way for a deeper understanding of the thermodynamics of multicomponent intracellular phase behaviour and its interplay with the nonequilibrium activity that is characteristic of endogenous condensates.
Conflict of interest statement
Declaration of Interests
R.W.K. is a consultant for and D.M.M. is recently employed by Dewpoint Therapeutics, LLC. The remaining authors have no financial or non-financial conflicts of interest.
Figures
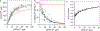



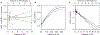




Comment in
-
Formation of liquid-like cellular organelles depends on their composition.Nature. 2020 May;581(7807):144-145. doi: 10.1038/d41586-020-01280-1. Nature. 2020. PMID: 32376935 No abstract available.
References
-
- Shin Y. & Brangwynne CP Liquid phase condensation in cell physiology and disease. Science 357, (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

