Rapid growth of new atmospheric particles by nitric acid and ammonia condensation
- PMID: 32405020
- PMCID: PMC7334196
- DOI: 10.1038/s41586-020-2270-4
Rapid growth of new atmospheric particles by nitric acid and ammonia condensation
Abstract
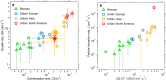
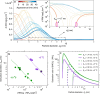
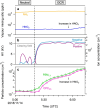
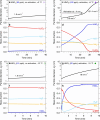
A list of authors and their affiliations appears at the end of the paper New-particle formation is a major contributor to urban smog1,2, but how it occurs in cities is often puzzling3. If the growth rates of urban particles are similar to those found in cleaner environments (1-10 nanometres per hour), then existing understanding suggests that new urban particles should be rapidly scavenged by the high concentration of pre-existing particles. Here we show, through experiments performed under atmospheric conditions in the CLOUD chamber at CERN, that below about +5 degrees Celsius, nitric acid and ammonia vapours can condense onto freshly nucleated particles as small as a few nanometres in diameter. Moreover, when it is cold enough (below -15 degrees Celsius), nitric acid and ammonia can nucleate directly through an acid-base stabilization mechanism to form ammonium nitrate particles. Given that these vapours are often one thousand times more abundant than sulfuric acid, the resulting particle growth rates can be extremely high, reaching well above 100 nanometres per hour. However, these high growth rates require the gas-particle ammonium nitrate system to be out of equilibrium in order to sustain gas-phase supersaturations. In view of the strong temperature dependence that we measure for the gas-phase supersaturations, we expect such transient conditions to occur in inhomogeneous urban settings, especially in wintertime, driven by vertical mixing and by strong local sources such as traffic. Even though rapid growth from nitric acid and ammonia condensation may last for only a few minutes, it is nonetheless fast enough to shepherd freshly nucleated particles through the smallest size range where they are most vulnerable to scavenging loss, thus greatly increasing their survival probability. We also expect nitric acid and ammonia nucleation and rapid growth to be important in the relatively clean and cold upper free troposphere, where ammonia can be convected from the continental boundary layer and nitric acid is abundant from electrical storms4,5.
Conflict of interest statement
The authors declare no competing interests.
Figures




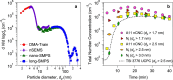
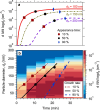

Comment in
-
Airborne particles might grow fast in cities.Nature. 2020 May;581(7807):145-146. doi: 10.1038/d41586-020-01334-4. Nature. 2020. PMID: 32405007 No abstract available.
References
-
- Stanier, C. O., Khlystov, A. Y. & Pandis, S. N. Nucleation events during the Pittsburgh Air Quality Study: description and relation to key meteorological, gas phase, and aerosol parameters. Aerosol Sci. Technol. 38, 253–264 (2004).
-
- Yao, L. et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science361, 278–281 (2018). - PubMed
-
- Kulmala, M., Kerminen, V.-M., Petäjä, T., Ding, A. J. & Wang, L. Atmospheric gas-to-particle conversion: why NPF events are observed in megacities? Faraday Discuss. 200, 271–288 (2017). - PubMed
-
- Höpfner, M. et al. Ammonium nitrate particles formed in upper troposphere from ground ammonia sources during Asian monsoons. Nat. Geosci. 12, 608–612 (2019).
-
- Williamson, C. J. et al. A large source of cloud condensation nuclei from new particle formation in the tropics. Nature574, 399–403 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

