Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors
- PMID: 32405062
- PMCID: PMC8127102
- DOI: 10.1038/s41591-020-0856-x
Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors
Abstract
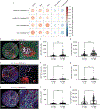
Serum interleukin-8 (IL-8) levels and tumor neutrophil infiltration are associated with worse prognosis in advanced cancers. Here, using a large-scale retrospective analysis, we show that elevated baseline serum IL-8 levels are associated with poor outcome in patients (n = 1,344) with advanced cancers treated with nivolumab and/or ipilimumab, everolimus or docetaxel in phase 3 clinical trials, revealing the importance of assessing serum IL-8 levels in identifying unfavorable tumor immunobiology and as an independent biomarker in patients receiving immune-checkpoint inhibitors.
Conflict of interest statement
Competing interests
I.M. reports receiving commercial research grants from Bristol-Myers Squibb, Bioncotech, Alligator, AstraZeneca and Roche; has received speakers bureau honoraria from MSD; and is a consultant/advisory board member for Bristol-Myers Squibb, Roche, Genmab, F-Star, Bioncotech, Bayer, Numab, Alligator, Boehringer Ingelheim and AstraZeneca. K.A.S. reports receiving research funding from Genoptix/Navigate (Novartis), Vasculox/Tioma, Tesaro, Moderna Therapeutics, Takeda, Surface Oncology, Pierre-Fabre Research Institute, Merck, Bristol-Myers Squibb, AstraZeneca and Eli Lilly; has received consultant/advisory board fees from Celgene, Moderna Therapeutics, Shattuck Labs, Pierre-Fabre, AbbVie, AstraZeneca, EMD Serono, Ono Pharmaceuticals, Clinica Alemana de Santiago, Dynamo Therapeutics, Torque Therapeutics, Agenus and Takeda; and has received speaker honoraria and/or travel support for meetings from Takeda, Merck, Bristol-Myers Squibb, Fluidigm, Peerview, Biotrac, Cambridge Healthtech Institute, Shattuck Labs and Genentech. J.L.P.-G. reports grants, personal fees and nonfinancial support from Roche, Bristol-Myers Squibb and MSD; and reports grants and personal fees from Ipsen and Eisai, and grants from Incyte and Janssen. M.C., M.Z., S.-P.H., T.C. and Y.F. have a patent (‘Combination Therapy with Anti-IL-8 Antibodies and Anti-PD-1 Antibodies for Treating Cancer’, publication number WO 2019140150). T.P.R. is a co-inventor in a provisional patent application (application number 62/650047) regarding the use of anti-IL-8 and anti-PD1 for the treatment of cancer. M.C., M.Z., T.C., Y.F., S.-P.H., A.M.W, V.B, D.P., T.B., D.L., T.P.R., P.P., J.G. and H.Z. are current or former employees of Bristol-Myers Squibb, which is developing anti-IL-8 (
Figures
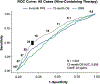

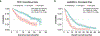

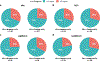
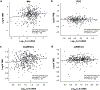


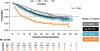

Comment in
-
IL-8 and cancer prognosis on immunotherapy.Nat Med. 2020 May;26(5):650-651. doi: 10.1038/s41591-020-0873-9. Nat Med. 2020. PMID: 32405061 No abstract available.
References
-
- O’Donnell JS, Teng MWL & Smyth MJ Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol 16, 151–167 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

