Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia
- PMID: 32405160
- PMCID: PMC7219361
- DOI: 10.1016/j.ejim.2020.05.009
Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia
Abstract
Background: Pneumonia with respiratory failure represents the main cause of death in COVID-19, where hyper inflammation plays an important role in lung damage. This study aims to evaluate if tocilizumab, an anti-soluble IL-6 receptor monoclonal antibody, reduces patients' mortality.
Methods: 85 consecutive patients admitted to the Montichiari Hospital (Italy) with COVID-19 related pneumonia and respiratory failure, not needing mechanical ventilation, were included if satisfying at least one among: respiratory rate ≥ 30 breaths/min, peripheral capillary oxygen saturation ≤ 93% or PaO2/FiO2<=300 mmHg. Patients admitted before March 13th (n=23) were prescribed the standard therapy (hydroxychloroquine, lopinavir and ritonavir) and were considered controls. On March 13th tocilizumab was available and patients admitted thereafter (n=62) received tocilizumab once within 4 days from admission, plus the standard care.
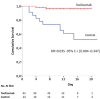
Results: Patients receiving tocilizumab showed significantly greater survival rate as compared to control patients (hazard ratio for death, 0.035; 95% confidence interval [CI], 0.004 to 0.347; p = 0.004), adjusting for baseline clinical characteristics. Two out of 62 patients of the tocilizumab group and 11 out of 23 in the control group died. 92% and 42.1% of the discharged patients in the tocilizumab and control group respectively, recovered. The respiratory function resulted improved in 64.8% of the observations in tocilizumab patients who were still hospitalized, whereas 100% of controls worsened and needed mechanical ventilation. No infections were reported.
Conclusions: Tocilizumab results to have a positive impact if used early during Covid-19 pneumonia with severe respiratory syndrome in terms of increased survival and favorable clinical course.
Keywords: COVID-19; Pneumonia; Respiratory failure; Retrospective study; SARS-cov-2; Tocilizumab.
Copyright © 2020 European Federation of Internal Medicine. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest No conflict of interest has been declared by any author.
Figures
Comment in
-
Tocilizumab for severe COVID-19: A promising intervention affecting inflammation and coagulation.Eur J Intern Med. 2020 Jun;76:21-22. doi: 10.1016/j.ejim.2020.05.018. Epub 2020 May 16. Eur J Intern Med. 2020. PMID: 32425364 Free PMC article. No abstract available.
References
-
- Liu, T., et al., The potential role of IL-6 in monitoring severe case of coronavirus disease2019. medRxiv.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

