NASH-inducing Diets in Göttingen Minipigs
- PMID: 32405177
- PMCID: PMC7212300
- DOI: 10.1016/j.jceh.2019.09.004
NASH-inducing Diets in Göttingen Minipigs
Abstract
Background: Owing to the human-like physiology, a minipig model of nonalcoholic steatohepatitis (NASH) could be valuable. Pigs, however, rarely develop substantial hepatic steatosis, even when fed diets with high fat, fructose, and cholesterol (FFC) content. The potential of choline-deficient, amino acid-defined high-fat diets (CDAHFD) was therefore evaluated in Göttingen Minipigs.
Methods: Castrated male Göttingen Minipigs were fed either chow (n = 5) or one of the three NASH diets: FFC (n = 5), CDAHFD with sucrose (CDAHFD-S; n = 4), or fructose (CDAHFD-F; n = 4) for 8 weeks. Liver and blood samples were collected after 2 weeks and at termination.
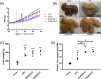
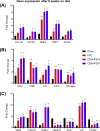
Results: Compared with chow, the body weight was higher after FFC (9.8 ± 0.4 versus 8.5 ± 1.2 kg; mean ± SD) and less after CDAHFD-S (6.4 ± 0.8 kg) and CDAHFD-F (6.9 ± 0.8 kg). Liver weight per kg body weight was significantly increased in all 3 NASH groups (FFC 2.1 times; and both CDAHFD diets 3.1 times). Histologically, pronounced macrovesicular steatosis developed only in the CDAHFD groups. Inflammation was present in all three NASH groups. In the CDAHFD groups, inflammatory cells formed crown-like structures around steatotic hepatocytes. Sirius red staining revealed mild fibrosis in the two CDAHFD groups with the fibrotic potential being further supported by immunohistochemical staining for activated stellate cells and gene expression analyses. No noticeable differences were found between CDAHFD-S and CDAHFD-F.
Conclusions: Göttingen Minipigs fed CDAHFD developed pronounced steatosis with inflammation around steatotic hepatocytes and incipient fibrosis, thereby showing potential as a model for human NASH. Further studies are needed to investigate the period needed for marked fibrosis to develop.
Keywords: -F, with fructose); ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; Animal model; CD45, cluster of differentiation 45; CDAHFD, choline-deficient; Choline; EDTA, ethylenediaminetetraacetic acid; FFC, high-fat, fructose, cholesterol; Fatty liver; Fibrosis; GGT, gamma-glutamyltransferase; GLDH, glutamate dehydrogenase; HE, hematoxylin and eosin; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; Porcine; SMA, smooth muscle actin; amino acid defined high-fat diet (-S, with sucrose.
© 2019 The Authors.
Figures




References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Ekstedt M., Hagstrom H., Nasr P. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. - PubMed
-
- Musso G., Cassader M., Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov. 2016;15:249–274. - PubMed
-
- Tsuchida T., Friedman S. Mechanism of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. - PubMed
-
- Dawson H.D., Smith A.D., Chen C., Urban J.F., Jr. An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Vet Microbiol. 2017;202:2–15. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

