Purification and characterization of a novel type of neurotoxic peptides from the venom of the Iranian scorpion Hemiscorpius lepturus
- PMID: 32405362
- PMCID: PMC7211352
- DOI: 10.22038/IJBMS.2019.37910.9025
Purification and characterization of a novel type of neurotoxic peptides from the venom of the Iranian scorpion Hemiscorpius lepturus
Abstract
Objectives: Scorpion venom has toxic effects on mammals, insects and crustaceans. Toxicogenic peptides are major contributors to the scorpion venom, which make it toxic. The Hemiscorpius lepturus (H. lepturus) is one of the most common scorpion bites agent, and responsible for 95% of scorpion bite deaths cases in Iran.
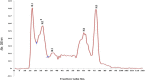
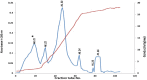
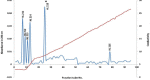
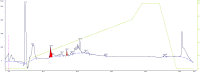
Materials and methods: In this project, we fractionated the H. lepturus scorpion venom and analyzed toxic fractions of the venom. The crude venom of H. lepturus was dialyzed against distilled water and then the soluble part of the venom was isolated from the non-soluble (mucoproteins) part of the venom and loaded onto the Sephadex G-50 gel filtration column, then after determining the toxicity of the obtained fractions (fractions toxicity were detected in mice by IV injection), the resulting toxic fraction was purified with three stages of ion-exchange chromatography (anion and cationic) and RP-HPLC. The purity of the fractions was verified by SDS-PAGE electrophoreses.
Results: The LD50 of H. lepturus venom was 177.01 µg/mouse. The crude venom had 7 detectable bands with molecular weights of 10-100 KDa and one band less than 10 KDa. Finally, after the different stages of chromatography, two HL2153 and HL2155 peaks were obtained from the RP-HPLC, which were depicted single bands and high purity. The electrophoretic analysis showed molecular weight 4874 Da for HL2153 and 5107 Da for HL2155 toxins.
Conclusion: It is concluded that H. lepturus venom contains two HL2153 and HL2155 toxins with a relatively similar molecular weight and similar electrical charge 4874 and 5107 Da, respectively.
Keywords: Hemiscorpius lepturus; Purification; Scorpion venom; Toxic peptides; Toxin.
Figures

References
-
- Radmanesh M. Cutaneous manifestations of the Hemiscorpius lepturus sting: a clinical study. Int J Dermatol. 1998;37:500–507. - PubMed
-
- Jalali A, Pipelzadeh MH, Sayedian R, Rowan E. A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran. Toxicon. 2010;55:173–179. - PubMed
-
- Radmanesh M. Clinical study of Hemiscorpion lepturus in Iran. J Trop Med Hyg. 1990;93:327–332. - PubMed
-
- Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nat Rev Drug Discov. 2003;2:790–802. - PubMed
-
- Torres-Larios A, Gurrola GB, Zamudio FZ, Possani LD. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur J Biochem. 2000;267:5023–5031. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
