A new antimicrobial compound from the leaves of Dodonaea viscosa for infectious diseases
- PMID: 32405575
- PMCID: PMC7210477
- DOI: 10.1016/j.bioactmat.2020.04.006
A new antimicrobial compound from the leaves of Dodonaea viscosa for infectious diseases
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Bioact Mater. 2020 Dec 4;6(6):1789-1790. doi: 10.1016/j.bioactmat.2020.11.009. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33336111 Free PMC article.
Abstract
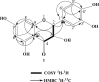
The main aim of this research work was to isolate and characterize the antimicrobial compounds that can be extracted from the leaves of Dodonaea viscosa (D. viscosa) and to assess their antimicrobial potency by established in-vitro agar diffusion method. The methanol extract was liquefied and fractioned by using a separatory funnel and organic solvents having different polarities. The agar diffusion technique was used to assess the antimicrobial potency of extracts and pure compounds against 5 g (+and -) microbial strains. Antimicrobial potency results showed that all extracts and isolated pure compounds provided significant antimicrobial potency against the applied microbial strains. The highest activity chloroform extract was analysed by column chromatography (CC) for the isolation of antimicrobial compounds. The structure of the isolated compounds was characterized based on 1D, 2D NMR and MS spectra. In conclusion, purest compounds might be useful as a remedy for infectious diseases.
Keywords: Antimicrobial compounds; Antimicrobial potency; Chromatography; Dodonaea viscosa; MS; NMR; Soxhlet.
© 2020 Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures






References
-
- M Aaerestrup F., Kane A.A., Cars O O. World health organization; Geneva, Switzerland: 2012. The Evolving Threat of Antimicrobial Resistance Options for Action.http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf Available at:
-
- Al-matani S.K., Al-Wahaibi R.N.S., Hossain M.A. In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev A Nat. Sci. Eng. 2015;17(3):103–108.
-
- Pretorius J.C., Magama S., Zietsman P.C., Van Wyk BE B.E. Growth inhibition of plant pathogenic bacteria and fungi by extracts from selected South African plant species. South Afr. J. Bot. 2009;69(2):186–192.
LinkOut - more resources
Full Text Sources

