Fast pulsatile blood flow measurement in deep tissue through a multimode detection fiber
- PMID: 32406214
- PMCID: PMC7219964
- DOI: 10.1117/1.JBO.25.5.055003
Fast pulsatile blood flow measurement in deep tissue through a multimode detection fiber
Abstract
Significance: Noninvasive in vivo fast pulsatile blood flow measurement in deep tissue is important because the blood flow waveform is correlated with physiological parameters, such as blood pressure and elasticity of blood vessels. Compromised blood flow may cause diseases, such as stroke, foot ulcer, and myocardial ischemia. There is great clinical demand for a portable and cost-effective device for noninvasive pulsatile blood flow measurement.
Aim: A diffuse-optics-based method, diffuse speckle pulsatile flowmetry (DSPF), was developed for fast measurement (∼300 Hz) of deep tissue blood flow noninvasively. To validate its performance, both a phantom experiment and in vivo demonstration were conducted.
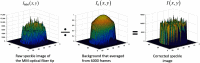
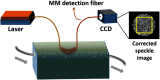
Approach: Over the past two decades, single-mode fibers have been used as detection fibers in most diffuse-optics-based deep tissue blood flow measurement modalities. We used a multimode (MM) detection fiber with a core size of 200 μm for diffused speckle pattern detection. A background intensity correction algorithm was implemented for speckle contrast calculation. The MM detection fiber helped to achieve a level of deep tissue blood flow measurement similar to that of conventional modalities, such as diffuse correlation spectroscopy and diffuse speckle contrast analysis, but it increases the measurement rate of blood flow to 300 Hz.
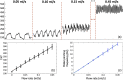
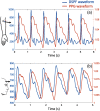
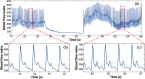
Results: The design and implementation of the DSPF system were introduced. The theory of the background intensity correction for the diffused speckle pattern detected by the MM fiber was explained. A flow phantom was built for validation of the performance of the DSPF system. An in vivo cuff-induced occlusion experiment was performed to demonstrate the capability of the proposed DSPF system.
Conclusions: An MM detection fiber can help to achieve fast (∼300 Hz) pulsatile blood flow measurement in the proposed DSPF method. The cost-effective device and the fiber-based flexible probe increase the usability of the DSPF system significantly.
Keywords: deep tissue blood flow; diffuse correlation spectroscopy; diffuse speckle contrast analysis; laser speckle.
Figures

Similar articles
-
Deep tissue flowmetry based on diffuse speckle contrast analysis.Opt Lett. 2013 May 1;38(9):1401-3. doi: 10.1364/OL.38.001401. Opt Lett. 2013. PMID: 23632498
-
A portable optical pulsatile flowmetry demonstrates strong clinical relevance for diabetic foot perfusion assessment.APL Bioeng. 2024 Feb 21;8(1):016109. doi: 10.1063/5.0182670. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38390315 Free PMC article.
-
Fiber-based laser speckle imaging for the detection of pulsatile flow.Lasers Surg Med. 2015 Aug;47(6):520-5. doi: 10.1002/lsm.22370. Lasers Surg Med. 2015. PMID: 26202900 Free PMC article.
-
Laser speckle contrast imaging: theoretical and practical limitations.J Biomed Opt. 2013 Jun;18(6):066018. doi: 10.1117/1.JBO.18.6.066018. J Biomed Opt. 2013. PMID: 23807512 Review.
-
[In vivo measurement of ocular circulation with the laser speckle method--development of apparatus and application in ophthalmological research].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):871-909. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643292 Review. Japanese.
Cited by
-
Impact of light polarization on laser speckle contrast imaging with a custom phantom for microvascular flow.Sci Rep. 2024 Nov 4;14(1):26652. doi: 10.1038/s41598-024-73757-2. Sci Rep. 2024. PMID: 39496642 Free PMC article.
-
Ultrasound Flow Imaging Study on Rat Brain with Ultrasound and Light Stimulations.Bioengineering (Basel). 2024 Feb 10;11(2):174. doi: 10.3390/bioengineering11020174. Bioengineering (Basel). 2024. PMID: 38391660 Free PMC article.
-
Anatomical Modeling and Optimization of Speckle Contrast Optical Tomography.bioRxiv [Preprint]. 2023 Sep 6:2023.09.06.556565. doi: 10.1101/2023.09.06.556565. bioRxiv. 2023. PMID: 37732196 Free PMC article. Preprint.
-
Interferometric diffusing wave spectroscopy imaging with an electronically variable time-of-flight filter.Optica. 2023 Jan 20;10(1):42-52. doi: 10.1364/optica.472471. Epub 2023 Jan 5. Optica. 2023. PMID: 37275218 Free PMC article.
-
An affordable, wearable, fiber-free pulsed-mode diffuse speckle contrast flowmetry (PM-DSCF) sensor for noninvasive measurements of deep cerebral blood flow.ArXiv [Preprint]. 2025 Feb 11:arXiv:2502.08000v1. ArXiv. 2025. PMID: 39990801 Free PMC article. Preprint.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

