Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications
- PMID: 32406215
- PMCID: PMC7219965
- DOI: 10.1117/1.JBO.25.7.071203
Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications
Abstract
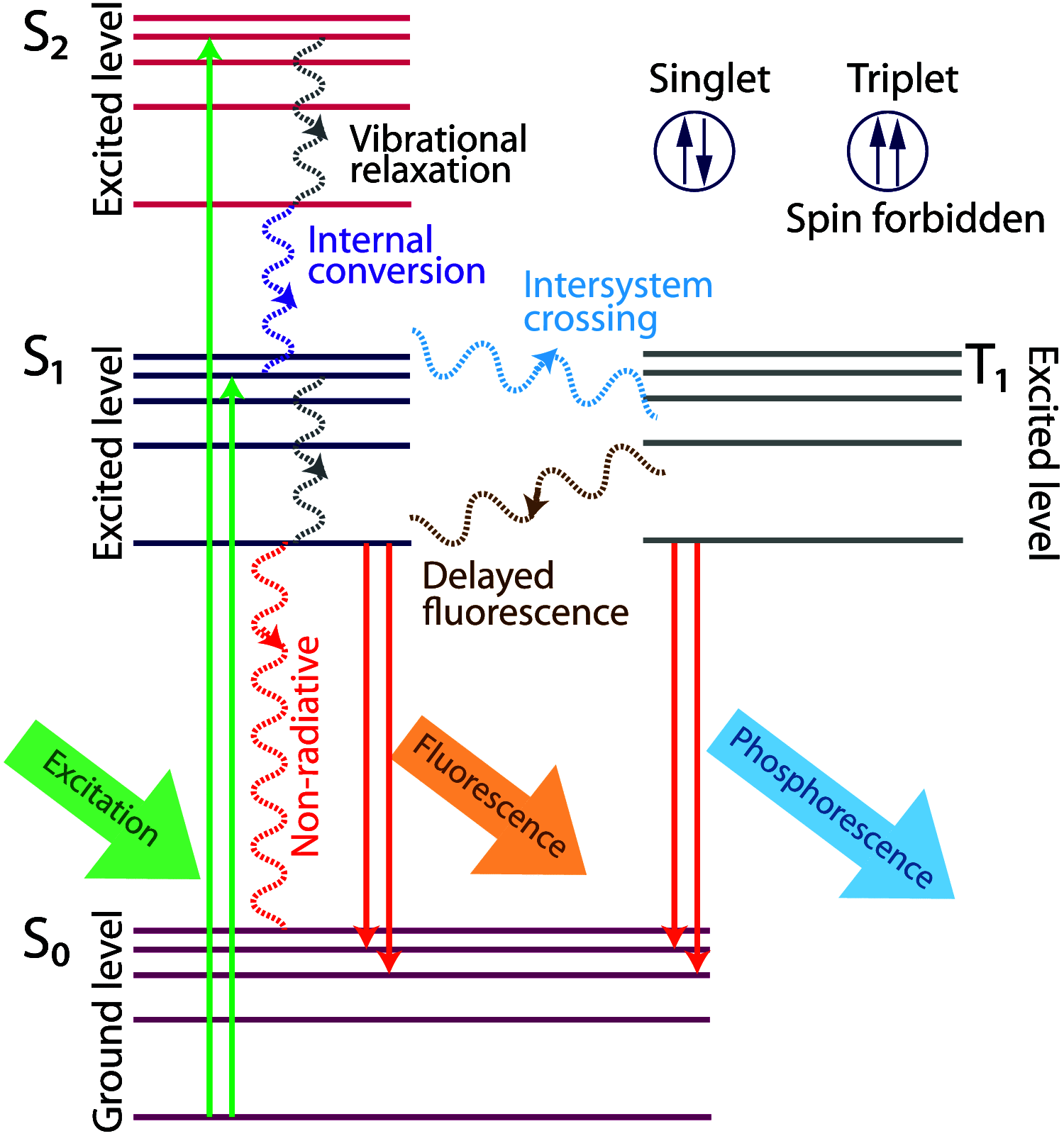
Significance: Fluorescence lifetime imaging microscopy (FLIM) is a powerful technique to distinguish the unique molecular environment of fluorophores. FLIM measures the time a fluorophore remains in an excited state before emitting a photon, and detects molecular variations of fluorophores that are not apparent with spectral techniques alone. FLIM is sensitive to multiple biomedical processes including disease progression and drug efficacy.
Aim: We provide an overview of FLIM principles, instrumentation, and analysis while highlighting the latest developments and biological applications.
Approach: This review covers FLIM principles and theory, including advantages over intensity-based fluorescence measurements. Fundamentals of FLIM instrumentation in time- and frequency-domains are summarized, along with recent developments. Image segmentation and analysis strategies that quantify spatial and molecular features of cellular heterogeneity are reviewed. Finally, representative applications are provided including high-resolution FLIM of cell- and organelle-level molecular changes, use of exogenous and endogenous fluorophores, and imaging protein-protein interactions with Förster resonance energy transfer (FRET). Advantages and limitations of FLIM are also discussed.
Conclusions: FLIM is advantageous for probing molecular environments of fluorophores to inform on fluorophore behavior that cannot be elucidated with intensity measurements alone. Development of FLIM technologies, analysis, and applications will further advance biological research and clinical assessments.
Keywords: cell heterogeneity; fluorescence lifetime; image analysis; microscopy; review.
Figures




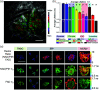
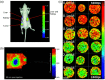
References
-
- Stokes G. G., “On the change of refrangibility of light,” Philos. Trans. R. Soc. Lond. 142, 463–562 (1852). 10.1098/rstl.1852.0022 - DOI
-
- Jameson D. M., Ed., Perspectives on Fluorescence: A Tribute to Gregorio Weber, Springer International Publishing, Switzerland: (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

