Two-Phase Synthesis of Taxol
- PMID: 32406238
- PMCID: PMC8349513
- DOI: 10.1021/jacs.0c03592
Two-Phase Synthesis of Taxol
Abstract
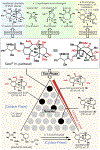
Taxol (a brand name for paclitaxel) is widely regarded as among the most famed natural isolates ever discovered, and has been the subject of innumerable studies in both basic and applied science. Its documented success as an anticancer agent, coupled with early concerns over supply, stimulated a furious worldwide effort from chemists to provide a solution for its preparation through total synthesis. Those pioneering studies proved the feasibility of retrosynthetically guided access to synthetic Taxol, albeit in minute quantities and with enormous effort. In practice, all medicinal chemistry efforts and eventual commercialization have relied upon natural (plant material) or biosynthetically derived (synthetic biology) supplies. Here we show how a complementary divergent synthetic approach that is holistically patterned off of biosynthetic machinery for terpene synthesis can be used to arrive at Taxol.
Figures

References
-
- Nicolaou KC; Montagnon T Molecules That Changed the World: A Brief History of the Art and Science of Synthesis and Its Impact on Society, 1st ed.; Wiley-VCH: Weinheim, 2008.
-
- Brickley P NIH Censured for Taxol Deal. Genome Biol. 2003, 4 (1).
-
- Celgene. Press Release Archive. 2020, https://ir.celgene.com/press-releases-archive/defa.
-
- Commerçon A; Bézard D; Bernard F; Bourzat JD Improved Protection and Esterification of a Precursor of the Taxotere® and Taxol Side Chains. Tetrahedron Lett. 1992, 33 (36), 5185–5188.
-
- Drug Approval Package: Taxotere (Docetaxel) #020449s028 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/20-449s028_Taxot... (accessed Feb 11, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

