Comparison of Human Platelet Lysate versus Fetal Bovine Serum for Expansion of Human Articular Cartilage-Derived Chondroprogenitors
- PMID: 32406256
- PMCID: PMC8804717
- DOI: 10.1177/1947603520918635
Comparison of Human Platelet Lysate versus Fetal Bovine Serum for Expansion of Human Articular Cartilage-Derived Chondroprogenitors
Abstract
Purpose: Articular chondroprogenitors, a suitable contender for cell-based therapy in cartilage repair, routinely employ fetal bovine serum (FBS) for expansion and differentiation. The possibility of transplant rejections or zoonoses transmissions raise a need for xeno-free alternatives. Use of human platelet lysate (hPL), a nutrient supplement abundant in growth factors, has not been reported for human chondroprogenitor expansion thus far. Our aim was to compare the biological profile of chondroprogenitors grown in hPL versus FBS.
Methods: Chondroprogenitors were isolated from 3 osteoarthritic knee joints. Following differential fibronectin adhesion assay, passage 0 cells grown in (a) 10% FBS and (b) 10% hPL were considered for assessment of growth kinetics, surface marker expression, gene expression, and trilineage differentiation. Latent transforming growth factor-β1 (TGFβ1) levels were also measured for each culture medium used.
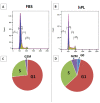
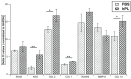
Results: Cellular proliferation was significantly higher in cells grown with hPL (P < 0.01). Surface marker expression was comparable except in CD-146 where hPL group had significantly higher values (P = 0.03). Comparison of mRNA expression revealed notably low values of collagen I, collagen X, aggrecan, and collagen II (P < 0.05). Trilineage differentiation was seen in both groups with higher alizarin red uptake noted in hPL. There were also significantly higher levels of latent TGFβ1 in the medium containing hPL as compared to FBS.
Conclusions: This is the first in vitro xeno-free study to affirm that hPL can serve as an optimal growth supplement for expansion of articular chondroprogenitors, although an in-depth assessment of resident growth factors and evaluation of different dilutions of hPL is required to assess suitability for use in translational research.
Keywords: chondroprogenitors; fetal bovine serum; human platelet lysate.
Conflict of interest statement
Figures





References
-
- Vinod E, Kachroo U, Amirtham SM, Ramasamy B, Sathishkumar S. Comparative analysis of fresh chondrocytes, cultured chondrocytes and chondroprogenitors derived from human articular cartilage. Acta Histochem. 2020;122(1):151462. - PubMed
-
- Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, et al.. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage. 2013;21:599-603. - PubMed
-
- Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325-38. - PubMed
-
- Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, et al.. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251-63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

