Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces
- PMID: 32406282
- PMCID: PMC7441755
- DOI: 10.1080/14712598.2020.1770222
Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces
Keywords: COVID-19; Chimeric antigen receptor (CAR) T cell‐induced cytokine release syndrome; anakinra; colchicine; coronavirus; inflammation; interleukin-6; macrophage activation syndrome; tocilizumab.
Figures
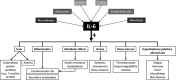


References
-
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020. April 29:202005615. [Epub ahead of print]. doi: 10.1073/pnas.2005615117. - DOI - PMC - PubMed
-
•• Outstanding report of an initial Chinese series of 20 patients with severe COVID-19 treated with tocilizumab with satisfactory results.
-
- Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020. May 3:102568. [Epub ahead of print]. - PMC - PubMed
-
•• A study-series of 100 consecutive patients with confirmed severe COVID-19 requiring ventilatory support who were treated with intravenous tocilizumab, obtaining rapid, sustained, and significant clinical improvement.
-
- Srirangan S, Choy EH.. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2:247–256. - PMC - PubMed
-
•• Excellent review on the role of IL-6 in the mechanisms associated with inflammation in rheumatoid arthritis. It describes in an elegant way the inflammatory pathways activated by IL-6.
-
- Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm. 2008;65:1413–1418. - PubMed
-
• In this report the author provided useful information on the pharmacology, pharmacokinetics, clinical efficacy, safety, and role of tocilizumab in rheumatoid arthritis
-
- Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. - PMC - PubMed
-
•• Review on the role of IL-6/JAK/STAT3 signalling in the tumor microenvironment and the status of preclinical and clinical investigations of agents targeting this pathway. The authors also discussed the potential of combining IL-6/JAK/STAT3 inhibitors with therapeutic agents directed against immune-checkpoint inhibitor.
Publication types
LinkOut - more resources
Full Text Sources
