The right place at the right time: Aurora B kinase localization to centromeres and kinetochores
- PMID: 32406506
- PMCID: PMC7805488
- DOI: 10.1042/EBC20190081
The right place at the right time: Aurora B kinase localization to centromeres and kinetochores
Abstract
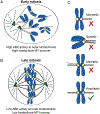
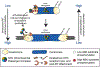
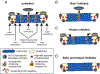
The fidelity of chromosome segregation during mitosis is intimately linked to the function of kinetochores, which are large protein complexes assembled at sites of centromeric heterochromatin on mitotic chromosomes. These key "orchestrators" of mitosis physically connect chromosomes to spindle microtubules and transduce forces through these connections to congress chromosomes and silence the spindle assembly checkpoint. Kinetochore-microtubule attachments are highly regulated to ensure that incorrect attachments are not prematurely stabilized, but instead released and corrected. The kinase activity of the centromeric protein Aurora B is required for kinetochore-microtubule destabilization during mitosis, but how the kinase acts on outer kinetochore substrates to selectively destabilize immature and erroneous attachments remains debated. Here, we review recent literature that sheds light on how Aurora B kinase is recruited to both centromeres and kinetochores and discuss possible mechanisms for how kinase interactions with substrates at distinct regions of mitotic chromosomes are regulated.
Keywords: Hec1; aurora kinases; cellular reproduction; kinetochores; microtubule; spindle.
© 2020 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Declaration of Interests
The authors declare that there are no competing interests regarding this manuscript.
Figures

Similar articles
-
Increased Aurora B activity causes continuous disruption of kinetochore-microtubule attachments and spindle instability.Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):E3996-4005. doi: 10.1073/pnas.1408017111. Epub 2014 Sep 8. Proc Natl Acad Sci U S A. 2014. PMID: 25201961 Free PMC article.
-
EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B.J Cell Biol. 2014 Mar 17;204(6):947-63. doi: 10.1083/jcb.201307119. Epub 2014 Mar 10. J Cell Biol. 2014. PMID: 24616220 Free PMC article.
-
Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells.J Cell Biol. 2020 Mar 2;219(3):e201905144. doi: 10.1083/jcb.201905144. J Cell Biol. 2020. PMID: 32028528 Free PMC article.
-
Regulation of kinetochore-microtubule attachments by Aurora B kinase.Biochem Soc Trans. 2009 Oct;37(Pt 5):976-80. doi: 10.1042/BST0370976. Biochem Soc Trans. 2009. PMID: 19754435 Review.
-
Establishing correct kinetochore-microtubule attachments in mitosis and meiosis.Essays Biochem. 2020 Sep 4;64(2):277-287. doi: 10.1042/EBC20190072. Essays Biochem. 2020. PMID: 32406497 Free PMC article. Review.
Cited by
-
Tension promotes kinetochore-microtubule release by Aurora B kinase.J Cell Biol. 2021 Jun 7;220(6):e202007030. doi: 10.1083/jcb.202007030. J Cell Biol. 2021. PMID: 33904910 Free PMC article.
-
Twenty years of merotelic kinetochore attachments: a historical perspective.Chromosome Res. 2023 Jul 19;31(3):18. doi: 10.1007/s10577-023-09727-7. Chromosome Res. 2023. PMID: 37466740 Free PMC article. Review.
-
Computational Biology Dynamics of Mps1 Kinase Molecular Interactions with Isoflavones Reveals a Chemical Scaffold with Potential to Develop New Therapeutics for the Treatment of Cancer.Int J Mol Sci. 2022 Nov 17;23(22):14228. doi: 10.3390/ijms232214228. Int J Mol Sci. 2022. PMID: 36430712 Free PMC article.
-
Polar Chromosomes-Challenges of a Risky Path.Cells. 2022 May 3;11(9):1531. doi: 10.3390/cells11091531. Cells. 2022. PMID: 35563837 Free PMC article. Review.
-
Solution conformational differences between conventional and CENP-A nucleosomes are accentuated by reversible deformation under high pressure.bioRxiv [Preprint]. 2025 Jan 21:2025.01.16.633457. doi: 10.1101/2025.01.16.633457. bioRxiv. 2025. Update in: Chromosome Res. 2025 Jun 12;33(1):11. doi: 10.1007/s10577-025-09769-z. PMID: 39896650 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

