Alcohol Cue-Induced Ventral Striatum Activity Predicts Subsequent Alcohol Self-Administration
- PMID: 32406553
- PMCID: PMC7336863
- DOI: 10.1111/acer.14342
Alcohol Cue-Induced Ventral Striatum Activity Predicts Subsequent Alcohol Self-Administration
Abstract
Background: Human laboratory paradigms are a pillar in medication development for alcohol use disorders (AUD). Neuroimaging paradigms, in which individuals are exposed to cues that elicit neural correlates of alcohol craving (e.g., mesocorticolimbic activation), are increasingly utilized to test the effects of AUD medications. Elucidation of the translational effects of these neuroimaging paradigms on human laboratory paradigms, such as self-administration, is warranted. The current study is a secondary analysis examining whether alcohol cue-induced activation in the ventral striatum is predictive of subsequent alcohol self-administration in the laboratory.
Methods: Non-treatment-seeking heavy drinkers of East Asian descent (n = 41) completed a randomized, placebo-controlled, double-blind, crossover experiment on the effects of naltrexone on neuroimaging and human laboratory paradigms. Participants completed 5 days of study medication (or placebo); on day 4, they completed a neuroimaging alcohol taste cue-reactivity task. On the following day (day 5), participants completed a 60-minute alcohol self-administration paradigm.
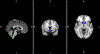
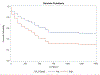
Results: Multilevel Cox regressions indicated a significant effect of taste cue-elicited ventral striatum activation on latency to first drink, Wald χ2 = 2.88, p = 0.05, such that those with higher ventral striatum activation exhibited shorter latencies to consume their first drink. Similarly, ventral striatum activation was positively associated with total number of drinks consumed, F(1, 38) = 5.90, p = 0.02. These effects were significant after controlling for alcohol use severity, OPRM1 genotype, and medication. Other potential regions of interest (anterior cingulate, thalamus) were not predictive of self-administration outcomes.
Conclusions: Neuroimaging alcohol taste cue paradigms may be predictive of laboratory paradigms such as self-administration. Elucidation of the relationships among different paradigms will inform how these paradigms may be used synergistically in experimental medicine and medication development.
Keywords: Alcohol Self-administration; Cue-Induced Craving; Human Laboratory; Neuroimaging; Ventral Striatum.
© 2020 by the Research Society on Alcoholism.
Figures
Similar articles
-
Pharmacogenetic Effects of Naltrexone in Individuals of East Asian Descent: Human Laboratory Findings from a Randomized Trial.Alcohol Clin Exp Res. 2018 Mar;42(3):613-623. doi: 10.1111/acer.13586. Epub 2018 Feb 1. Alcohol Clin Exp Res. 2018. PMID: 29265379 Free PMC article. Clinical Trial.
-
Nalmefene attenuates neural alcohol cue-reactivity in the ventral striatum and subjective alcohol craving in patients with alcohol use disorder.Psychopharmacology (Berl). 2021 Aug;238(8):2179-2189. doi: 10.1007/s00213-021-05842-7. Epub 2021 Apr 12. Psychopharmacology (Berl). 2021. PMID: 33846866 Free PMC article. Clinical Trial.
-
Neuroimaging findings from an experimental pharmacology trial of naltrexone in heavy drinkers of East Asian descent.Drug Alcohol Depend. 2019 Jul 1;200:181-190. doi: 10.1016/j.drugalcdep.2019.02.028. Epub 2019 May 9. Drug Alcohol Depend. 2019. PMID: 31160146 Free PMC article. Clinical Trial.
-
Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta-analysis.Addict Biol. 2019 Nov;24(6):1138-1152. doi: 10.1111/adb.12747. Epub 2019 May 30. Addict Biol. 2019. PMID: 31148304 Free PMC article.
-
Testing pharmacotherapies for alcohol use disorder with cue exposure paradigms: A systematic review and quantitative synthesis of human laboratory trial methodology.Alcohol Clin Exp Res (Hoboken). 2023 Sep;47(9):1629-1645. doi: 10.1111/acer.15143. Epub 2023 Jul 23. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 37423771 Free PMC article. Review.
Cited by
-
Operant ethanol self-administration behaviors do not predict sex differences in continuous access home cage drinking.Alcohol. 2025 Mar;123:87-99. doi: 10.1016/j.alcohol.2024.08.004. Epub 2024 Aug 31. Alcohol. 2025. PMID: 39218047
-
Regulation of alcohol drinking by ventral striatum and extended amygdala circuitry.Neuropharmacology. 2022 Jul 1;212:109074. doi: 10.1016/j.neuropharm.2022.109074. Epub 2022 Apr 26. Neuropharmacology. 2022. PMID: 35487273 Free PMC article. Review.
-
Potential biomarker of brain response to opioid antagonism in adolescents with eating disorders: a pilot study.Front Psychiatry. 2023 Jul 10;14:1161032. doi: 10.3389/fpsyt.2023.1161032. eCollection 2023. Front Psychiatry. 2023. PMID: 37492067 Free PMC article.
-
FMRI-based prediction of naltrexone response in alcohol use disorder: a replication study.Eur Arch Psychiatry Clin Neurosci. 2021 Aug;271(5):915-927. doi: 10.1007/s00406-021-01259-7. Epub 2021 Apr 21. Eur Arch Psychiatry Clin Neurosci. 2021. PMID: 33884495 Free PMC article.
-
Neural, Motivational, and Psychological Measures of Pain Avoidance Predict Future Alcohol Use in Adult Drinkers.Addict Biol. 2025 Feb;30(2):e70020. doi: 10.1111/adb.70020. Addict Biol. 2025. PMID: 39952897 Free PMC article.
References
-
- Allen JP, Litten RZ, Fertig JB, Babor T (1997) A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 21:613–619. - PubMed
-
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstadt-Klein S, Mann K, Perez-Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F, Sommer WH (2019) Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol. - PubMed
-
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A (2012) Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69:842–852. - PubMed

