In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables†
- PMID: 32406908
- PMCID: PMC7442777
- DOI: 10.1093/biolre/ioaa073
In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables†
Abstract
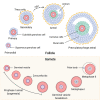
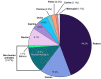
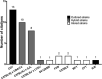
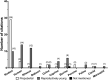
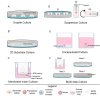
Folliculogenesis is a complex process that requires integration of autocrine, paracrine, and endocrine factors together with tightly regulated interactions between granulosa cells and oocytes for the growth and survival of healthy follicles. Culture of ovarian follicles is a powerful approach for investigating folliculogenesis and oogenesis in a tightly controlled environment. This method has not only enabled unprecedented insight into the fundamental biology of follicle development but also has far-reaching translational applications, including in fertility preservation for women whose ovarian follicles may be damaged by disease or its treatment or in wildlife conservation. Two- and three-dimensional follicle culture systems have been developed and are rapidly evolving. It is clear from a review of the literature on isolated follicle culture methods published over the past two decades (1980-2018) that protocols vary with respect to species examined, follicle isolation methods, culture techniques, culture media and nutrient and hormone supplementation, and experimental endpoints. Here we review the heterogeneity among these major variables of follicle culture protocols.
Keywords: follicle; follicle culture; follicular development; oocyte.
© The Author(s) 2020. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
In vitro growth of the ovarian follicle: taking stock of advances in research.JBRA Assist Reprod. 2022 Aug 4;26(3):508-521. doi: 10.5935/1518-0557.20210076. JBRA Assist Reprod. 2022. PMID: 34995044 Free PMC article. Review.
-
Recapitulating folliculogenesis and oogenesis outside the body: encapsulated in vitro follicle growth†.Biol Reprod. 2023 Jan 14;108(1):5-22. doi: 10.1093/biolre/ioac176. Biol Reprod. 2023. PMID: 36136744 Free PMC article.
-
Primate follicular development and oocyte maturation in vitro.Adv Exp Med Biol. 2013;761:43-67. doi: 10.1007/978-1-4614-8214-7_5. Adv Exp Med Biol. 2013. PMID: 24097381 Free PMC article. Review.
-
Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture.Hum Reprod. 2016 Jul;31(7):1531-9. doi: 10.1093/humrep/dew049. Epub 2016 Apr 24. Hum Reprod. 2016. PMID: 27112699
-
The earliest stages of folliculogenesis in vitro.Reproduction. 2002 Feb;123(2):185-202. doi: 10.1530/rep.0.1230185. Reproduction. 2002. PMID: 11866686 Review.
Cited by
-
New hyaluronan-based biomatrix for 3-D follicle culture yields functionally competent oocytes.Reprod Biol Endocrinol. 2022 Oct 10;20(1):148. doi: 10.1186/s12958-022-01019-9. Reprod Biol Endocrinol. 2022. PMID: 36217168 Free PMC article.
-
Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization.Bioengineering (Basel). 2024 Jul 15;11(7):719. doi: 10.3390/bioengineering11070719. Bioengineering (Basel). 2024. PMID: 39061801 Free PMC article.
-
Comparative study of the three-dimensional genomes of granulosa cells in germinal vesicle and metaphase II follicles.Front Genet. 2024 Nov 20;15:1480153. doi: 10.3389/fgene.2024.1480153. eCollection 2024. Front Genet. 2024. PMID: 39634272 Free PMC article.
-
Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction.Bioengineering (Basel). 2023 Jul 13;10(7):832. doi: 10.3390/bioengineering10070832. Bioengineering (Basel). 2023. PMID: 37508859 Free PMC article. Review.
-
In vitro growth of the ovarian follicle: taking stock of advances in research.JBRA Assist Reprod. 2022 Aug 4;26(3):508-521. doi: 10.5935/1518-0557.20210076. JBRA Assist Reprod. 2022. PMID: 34995044 Free PMC article. Review.
References
-
- Green LJ, Shikanov A. In vitro culture methods of preantral follicles. Theriogenology 2016; 86:229–238. - PubMed
-
- Matzuk MM, Burns KH. Genetics of mammalian reproduction: modeling the end of the germline. Annu Rev Physiol 2012; 74:503–528. - PubMed
-
- Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab 2018; 29:313–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

