PvrA is a novel regulator that contributes to Pseudomonas aeruginosa pathogenesis by controlling bacterial utilization of long chain fatty acids
- PMID: 32406921
- PMCID: PMC7293031
- DOI: 10.1093/nar/gkaa377
PvrA is a novel regulator that contributes to Pseudomonas aeruginosa pathogenesis by controlling bacterial utilization of long chain fatty acids
Abstract
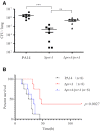
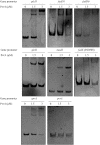
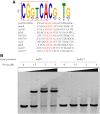
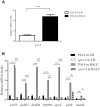
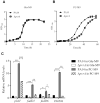
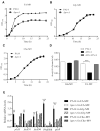
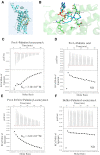
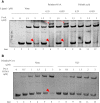
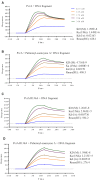
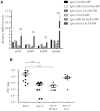
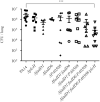
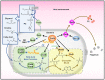
During infection of a host, Pseudomonas aeruginosa orchestrates global gene expression to adapt to the host environment and counter the immune attacks. P. aeruginosa harbours hundreds of regulatory genes that play essential roles in controlling gene expression. However, their contributions to the bacterial pathogenesis remain largely unknown. In this study, we analysed the transcriptomic profile of P. aeruginosa cells isolated from lungs of infected mice and examined the roles of upregulated regulatory genes in bacterial virulence. Mutation of a novel regulatory gene pvrA (PA2957) attenuated the bacterial virulence in an acute pneumonia model. Chromatin immunoprecipitation (ChIP)-Seq and genetic analyses revealed that PvrA directly regulates genes involved in phosphatidylcholine utilization and fatty acid catabolism. Mutation of the pvrA resulted in defective bacterial growth when phosphatidylcholine or palmitic acid was used as the sole carbon source. We further demonstrated that palmitoyl coenzyme A is a ligand for the PvrA, enhancing the binding affinity of PvrA to its target promoters. An arginine residue at position 136 was found to be essential for PvrA to bind palmitoyl coenzyme A. Overall, our results revealed a novel regulatory pathway that controls genes involved in phosphatidylcholine and fatty acid utilization and contributes to the bacterial virulence.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Similar articles
-
Regulatory and structural mechanisms of PvrA-mediated regulation of the PQS quorum-sensing system and PHA biosynthesis in Pseudomonas aeruginosa.Nucleic Acids Res. 2023 Apr 11;51(6):2691-2708. doi: 10.1093/nar/gkad059. Nucleic Acids Res. 2023. PMID: 36744476 Free PMC article.
-
Pseudomonas aeruginosa regulator PvrA binds simultaneously to multiple pseudo-palindromic sites for efficient transcription activation.Sci China Life Sci. 2024 May;67(5):900-912. doi: 10.1007/s11427-022-2363-y. Epub 2023 Sep 26. Sci China Life Sci. 2024. PMID: 37938507
-
SuhB is a regulator of multiple virulence genes and essential for pathogenesis of Pseudomonas aeruginosa.mBio. 2013 Oct 29;4(6):e00419-13. doi: 10.1128/mBio.00419-13. mBio. 2013. PMID: 24169572 Free PMC article.
-
Pseudomonas aeruginosa AlgR Phosphorylation Status Differentially Regulates Pyocyanin and Pyoverdine Production.mBio. 2018 Jan 30;9(1):e02318-17. doi: 10.1128/mBio.02318-17. mBio. 2018. PMID: 29382736 Free PMC article.
-
Regulation of alginate gene expression in Pseudomonas aeruginosa.Methods Enzymol. 1994;235:493-502. doi: 10.1016/0076-6879(94)35165-1. Methods Enzymol. 1994. PMID: 8057921 Review. No abstract available.
Cited by
-
Revisiting long-chain fatty acid metabolism in Escherichia coli: integration with stress responses.Curr Genet. 2021 Aug;67(4):573-582. doi: 10.1007/s00294-021-01178-z. Epub 2021 Mar 19. Curr Genet. 2021. PMID: 33740112 Review.
-
LipR functions as an intracellular pH regulator in Bacillus thuringiensis under glucose conditions.mLife. 2023 Feb 11;2(1):58-72. doi: 10.1002/mlf2.12055. eCollection 2023 Mar. mLife. 2023. PMID: 38818337 Free PMC article.
-
High-Level Expression of Cell-Surface Signaling System Hxu Enhances Pseudomonas aeruginosa Bloodstream Infection.Infect Immun. 2022 Oct 20;90(10):e0032922. doi: 10.1128/iai.00329-22. Epub 2022 Sep 28. Infect Immun. 2022. PMID: 36169312 Free PMC article.
-
MITF Contributes to the Body Color Differentiation of Sea Cucumbers Apostichopus japonicus through Expression Differences and Regulation of Downstream Genes.Biology (Basel). 2022 Dec 20;12(1):1. doi: 10.3390/biology12010001. Biology (Basel). 2022. PMID: 36671694 Free PMC article.
-
Transcriptional Regulators Controlling Virulence in Pseudomonas aeruginosa.Int J Mol Sci. 2023 Jul 25;24(15):11895. doi: 10.3390/ijms241511895. Int J Mol Sci. 2023. PMID: 37569271 Free PMC article. Review.
References
-
- Lyczak J.B., Cannon C.L., Pier G.B.. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000; 2:1051–1060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

