In vivo assessment of respiratory burst inhibition by xenobiotic exposure using larval zebrafish
- PMID: 32407153
- PMCID: PMC7405951
- DOI: 10.1080/1547691X.2020.1748772
In vivo assessment of respiratory burst inhibition by xenobiotic exposure using larval zebrafish
Abstract
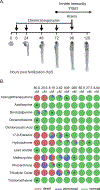
Currently, assessment of the potential immunotoxicity of a given agent involves a tiered approach for hazard identification and mechanistic studies, including observational studies, evaluation of immune function, and measurement of susceptibility to infectious and neoplastic diseases. These studies generally use costly low-throughput mammalian models. Zebrafish, however, offer an excellent alternative due to their rapid development, ease of maintenance, and homology to mammalian immune system function and development. Larval zebrafish also are a convenient model to study the innate immune system with no interference from the adaptive immune system. In this study, a respiratory burst assay (RBA) was utilized to measure reactive oxygen species (ROS) production after developmental xenobiotic exposure. Embryos were exposed to non-teratogenic doses of chemicals and at 96 h post-fertilization, the ability to produce ROS was measured. Using the RBA, 12 compounds with varying immune-suppressive properties were screened. Seven compounds neither suppressed nor enhanced the respiratory burst; five reproducibly suppressed global ROS production, but with varying potencies: benzo[a]pyrene, 17β-estradiol, lead acetate, methoxychlor, and phenanthrene. These five compounds have all previously been reported as immunosuppressive in mammalian innate immunity assays. To evaluate whether the suppression of ROS by these compounds was a result of decreased immune cell numbers, flow cytometry with transgenic zebrafish larvae was used to count the numbers of neutrophils and macrophages after chemical exposure. With this assay, benzo[a]pyrene was found to be the only chemical that induced a change in the number of immune cells by increasing macrophage but not neutrophil numbers. Taken together, this work demonstrates the utility of zebrafish larvae as a vertebrate model for identifying compounds that impact innate immune function at non-teratogenic levels and validates measuring ROS production and phagocyte numbers as metrics for monitoring how xenobiotic exposure alters the innate immune system.
Keywords: Chemical screen; endocrine disrupting compounds (EDC); high throughput; lead; phagocyte; polycyclic aromatic hydrocarbons (PAH); reactive oxygen species (ROS).
Conflict of interest statement
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content of this manuscript.
Figures


References
-
- Astin J, Keerthisinghe P, Du L, Sanderson L, Crosier K, Crosier P, Hall C. 2017. Innate immune cells and bacterial infection in zebrafish. Meth. Cell Biol 138:31–60. - PubMed
-
- Bogdan C, Röllinghoff M, Diefenbach A. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific Immunity. Curr. Opin. Immunol 12:64–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
