Ovulation and ovarian wound healing are impaired with advanced reproductive age
- PMID: 32407290
- PMCID: PMC7288922
- DOI: 10.18632/aging.103237
Ovulation and ovarian wound healing are impaired with advanced reproductive age
Abstract
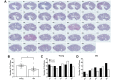
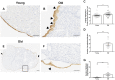
Aging is associated with reduced tissue remodeling efficiency and increased fibrosis, characterized by excess collagen accumulation and altered matrix degradation. Ovulation, the process by which an egg is released from the ovary, is one of the most dynamic cycles of tissue wounding and repair. Because the ovary is one of the first organs to age, ovulation and ovarian wound healing is impaired with advanced reproductive age. To test this hypothesis, we induced superovulation in reproductively young and old mice and determined the numbers of eggs ovulated and corpora lutea (CLs), the progesterone producing glands formed post-ovulation. Reproductively old mice ovulated fewer eggs and had fewer CLs relative to young controls. Moreover, reproductively old mice exhibited a greater number of oocytes trapped within CLs and expanded cumulus oocyte complexes within unruptured antral follicles, indicative of failed ovulation. In addition, post-ovulatory tissue remodeling was compromised with age as evidenced by reduced CL vasculature, increased collagen, decreased hyaluronan, decreased cell proliferation and apoptosis, impaired wound healing capacity, and aberrant morphology of the ovarian surface epithelium (OSE). These findings demonstrate that ovulatory dysfunction is an additional mechanism underlying the age-related loss of fertility beyond the reduction of egg quantity and quality.
Keywords: fibrosis; ovary; ovulation; reproductive aging; wound healing.
Conflict of interest statement
Figures












References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

