Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B
- PMID: 32407293
- PMCID: PMC7410053
- DOI: 10.1172/JCI130324
Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B
Abstract
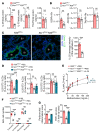
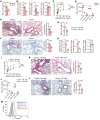
Mothers living near high-traffic roads before or during pregnancy are more likely to have children with asthma. Mechanisms are unknown. Using a mouse model, here we showed that maternal exposure to diesel exhaust particles (DEP) predisposed offspring to allergic airway disease (AAD, murine counterpart of human asthma) through programming of their NK cells; predisposition to AAD did not develop in DEP pups that lacked NK cells and was induced in normal pups receiving NK cells from WT DEP pups. DEP NK cells expressed GATA3 and cosecreted IL-13 and the killer protease granzyme B in response to allergen challenge. Extracellular granzyme B did not kill, but instead stimulated protease-activated receptor 2 (PAR2) to cooperate with IL-13 in the induction of IL-25 in airway epithelial cells. Through loss-of-function and reconstitution experiments in pups, we showed that NK cells and granzyme B were required for IL-25 induction and activation of the type 2 immune response and that IL-25 mediated NK cell effects on type 2 response and AAD. Finally, experiments using human cord blood and airway epithelial cells suggested that DEP might induce an identical pathway in humans. Collectively, we describe an NK cell-dependent endotype of AAD that emerged in early life as a result of maternal exposure to DEP.
Keywords: Asthma; Immunology; Mouse models; NK cells; Pulmonology.
Conflict of interest statement
Figures









References
-
- von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149(2 pt 1):358–364. - PubMed

