Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction
- PMID: 32407295
- PMCID: PMC7410081
- DOI: 10.1172/JCI122237
Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction
Abstract
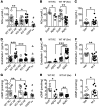
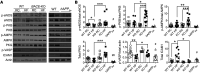
Diabetes, obesity, and Alzheimer's disease (AD) are associated with vascular complications and impaired nitric oxide (NO) production. Furthermore, increased β-site amyloid precursor protein-cleaving (APP-cleaving) enzyme 1 (BACE1), APP, and β-amyloid (Aβ) are linked with vascular disease development and increased BACE1 and Aβ accompany hyperglycemia and hyperlipidemia. However, the causal relationship between obesity and diabetes, increased Aβ, and vascular dysfunction is unclear. We report that diet-induced obesity (DIO) in mice increased plasma and vascular Aβ42 that correlated with decreased NO bioavailability, endothelial dysfunction, and increased blood pressure. Genetic or pharmacological reduction of BACE1 activity and Aβ42 prevented and reversed, respectively, these outcomes. In contrast, expression of human mutant APP in mice or Aβ42 infusion into control diet-fed mice to mimic obese levels impaired NO production, vascular relaxation, and raised blood pressure. In humans, increased plasma Aβ42 correlated with diabetes and endothelial dysfunction. Mechanistically, higher Aβ42 reduced endothelial NO synthase (eNOS), cyclic GMP (cGMP), and protein kinase G (PKG) activity independently of diet, whereas endothelin-1 was increased by diet and Aβ42. Lowering Aβ42 reversed the DIO deficit in the eNOS/cGMP/PKG pathway and decreased endothelin-1. Our findings suggest that BACE1 inhibitors may have therapeutic value in the treatment of vascular disease associated with diabetes.
Keywords: Endocrinology; Nitric oxide; Obesity; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures





Similar articles
-
Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid.J Cereb Blood Flow Metab. 2020 Feb;40(2):392-403. doi: 10.1177/0271678X18822474. Epub 2019 Jan 7. J Cereb Blood Flow Metab. 2020. PMID: 30614363 Free PMC article.
-
Endothelial nitric oxide modulates expression and processing of amyloid precursor protein.Circ Res. 2010 Dec 10;107(12):1498-502. doi: 10.1161/CIRCRESAHA.110.233080. Epub 2010 Dec 2. Circ Res. 2010. PMID: 21127294 Free PMC article.
-
Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding.Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):758-65. doi: 10.1161/ATVBAHA.109.199893. Epub 2010 Jan 21. Arterioscler Thromb Vasc Biol. 2010. PMID: 20093624 Free PMC article.
-
[The Role of GRK2 and Its Potential as a New Therapeutic Target in Diabetic Vascular Complications].Yakugaku Zasshi. 2015;135(8):961-7. doi: 10.1248/yakushi.15-00119. Yakugaku Zasshi. 2015. PMID: 26234354 Review. Japanese.
-
Endothelial nitric oxide: protector of a healthy mind.Eur Heart J. 2014 Apr;35(14):888-94. doi: 10.1093/eurheartj/eht544. Epub 2013 Dec 18. Eur Heart J. 2014. PMID: 24357508 Free PMC article. Review.
Cited by
-
The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics.Acta Neuropathol. 2020 Oct;140(4):417-447. doi: 10.1007/s00401-020-02196-w. Epub 2020 Jul 29. Acta Neuropathol. 2020. PMID: 32728795 Free PMC article. Review.
-
Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction.J Transl Med. 2022 Mar 22;20(1):141. doi: 10.1186/s12967-021-03220-7. J Transl Med. 2022. PMID: 35317838 Free PMC article. Review.
-
Inactivation of BACE1 increases expression of endothelial nitric oxide synthase in cerebrovascular endothelium.J Cereb Blood Flow Metab. 2022 Oct;42(10):1920-1932. doi: 10.1177/0271678X221105683. Epub 2022 Jun 8. J Cereb Blood Flow Metab. 2022. PMID: 35673977 Free PMC article.
-
How to measure and model cardiovascular aging.Cardiovasc Res. 2025 Aug 28;121(10):1489-1508. doi: 10.1093/cvr/cvaf138. Cardiovasc Res. 2025. PMID: 40873304 Free PMC article. Review.
-
Extracellular vesicle miR-32 derived from macrophage promotes arterial calcification in mice with type 2 diabetes via inhibiting VSMC autophagy.J Transl Med. 2022 Jul 6;20(1):307. doi: 10.1186/s12967-022-03502-8. J Transl Med. 2022. PMID: 35794619 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

