The role of the C2A domain of synaptotagmin 1 in asynchronous neurotransmitter release
- PMID: 32407359
- PMCID: PMC7224543
- DOI: 10.1371/journal.pone.0232991
The role of the C2A domain of synaptotagmin 1 in asynchronous neurotransmitter release
Abstract
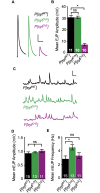
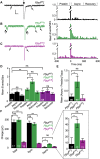
Following nerve stimulation, there are two distinct phases of Ca2+-dependent neurotransmitter release: a fast, synchronous release phase, and a prolonged, asynchronous release phase. Each of these phases is tightly regulated and mediated by distinct mechanisms. Synaptotagmin 1 is the major Ca2+ sensor that triggers fast, synchronous neurotransmitter release upon Ca2+ binding by its C2A and C2B domains. It has also been implicated in the inhibition of asynchronous neurotransmitter release, as blocking Ca2+ binding by the C2A domain of synaptotagmin 1 results in increased asynchronous release. However, the mutation used to block Ca2+ binding in the previous experiments (aspartate to asparagine mutations, sytD-N) had the unintended side effect of mimicking Ca2+ binding, raising the possibility that the increase in asynchronous release was directly caused by ostensibly constitutive Ca2+ binding. Thus, rather than modulating an asynchronous sensor, sytD-N may be mimicking one. To directly test the C2A inhibition hypothesis, we utilized an alternate C2A mutation that we designed to block Ca2+ binding without mimicking it (an aspartate to glutamate mutation, sytD-E). Analysis of both the original sytD-N mutation and our alternate sytD-E mutation at the Drosophila neuromuscular junction showed differential effects on asynchronous release, as well as on synchronous release and the frequency of spontaneous release. Importantly, we found that asynchronous release is not increased in the sytD-E mutant. Thus, our work provides new mechanistic insight into synaptotagmin 1 function during Ca2+-evoked synaptic transmission and demonstrates that Ca2+ binding by the C2A domain of synaptotagmin 1 does not inhibit asynchronous neurotransmitter release in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Striegel AR, Biela LM, Evans CS, Wang Z, Delehoy JB, Sutton RB, et al. Calcium binding by synaptotagmin’s C2A domain is an essential element of the electrostatic switch that triggers synchronous synaptic transmission. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(4):1253–60. Epub 2012/01/27. 10.1523/jneurosci.4652-11.2012 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

