The impact of rumen-protected amino acids on the expression of key- genes involved in the innate immunity of dairy sheep
- PMID: 32407360
- PMCID: PMC7224535
- DOI: 10.1371/journal.pone.0233192
The impact of rumen-protected amino acids on the expression of key- genes involved in the innate immunity of dairy sheep
Abstract
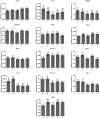
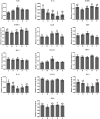
Rumen protected amino acids inclusion in ewes' diets has been proposed to enhance their innate immunity. The objective of this work was to determine the impact of dietary supplementation with rumen-protected methionine or lysine, as well as with a combination of these amino acids in two different ratios, on the expression of selected key-genes (NLRs, MyD88, TRIF, MAPK-1, IRF-3, JunD, TRAF-3, IRF-5, IL-1α, IL-10, IKK-α, STAT-3 and HO-1). Thus, sixty Chios dairy ewes (Ovis aries) were assigned to one of the following five dietary treatments (12 animals/ treatment): A: basal diet consist of concentrates, wheat straw and alfalfa hay (control group); B: basal diet +6.0 g/head rumen-protected methionine; C: basal diet + 5.0 g/head rumen-protected lysine; D: basal diet +6.0 g/head rumen-protected methionine + 5.0 g/head rumen-protected lysine and E: basal diet +12.0 g/head rumen-protected methionine + 5.0 g/head rumen-protected lysine. The results revealed a significant downregulation of relative transcript level of the IL-1α gene in the neutrophils of C and in monocytes of D ewes compared with the control. Significantly lower mRNA transcript accumulation was also observed for the MyD88 gene in the neutrophils of ewes fed with lysine only (C). The mRNA relative expression levels of JunD gene were highly induced in the monocytes, while those of IL-10 and HO-1 genes were declined in the neutrophils of ewes fed with the C and D diets compared with the control. Lower transcript levels of STAT-3 gene were observed in the neutrophils of ewes fed with either C or with E diets in comparison with the control. In conclusion, our results suggest that the dietary supplementation of ewes with rumen-protected amino acids, down regulate the expression of some genes involved in the pro-inflammatory signalling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Celi P, Chauhan SS. Oxidative stress management in farm animals: opportunities and challenges In ‘Proceedings of the 4th International Conference on Sustainable Animal Agriculture for Developing Countries (SAADC). 2013; 95–109
-
- Bertoni G, Minuti A, Trevisi E. Immune system inflammation and nutrition in dairy cattle. Anim. Prod. Sci. 2015; 55: 354–360. 10.1071/AN14863 - DOI
-
- Jacometo CB, Alharthi AS, Zhou Z, Luchini D, Loor JJ. Maternal supply of methionine during late pregnancy is associated with changes in immune function and abundance of microRNA and mRNA in Holstein calf polymorphonuclear leukocytes. J. Dairy Sci. 2018; 101(9):8146–8158. 10.3168/jds.2018-14428 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

