Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities
- PMID: 32407385
- PMCID: PMC7224462
- DOI: 10.1371/journal.ppat.1008508
Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities
Abstract
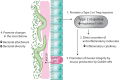
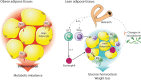
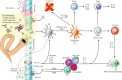
Parasitic helminths have coevolved with humans over millennia, intricately refining and developing an array of mechanisms to suppress or skew the host's immune system, thereby promoting their long-term survival. Some helminths, such as hookworms, cause little to no overt pathology when present in modest numbers and may even confer benefits to their human host. To exploit this evolutionary phenomenon, clinical trials of human helminth infection have been established and assessed for safety and efficacy for a range of immune dysfunction diseases and have yielded mixed outcomes. Studies of live helminth therapy in mice and larger animals have convincingly shown that helminths and their excretory/secretory products possess anti-inflammatory drug-like properties and represent an untapped pharmacopeia. These anti-inflammatory moieties include extracellular vesicles, proteins, glycans, post-translational modifications, and various metabolites. Although the concept of helminth-inspired therapies holds promise, it also presents a challenge to the drug development community, which is generally unfamiliar with foreign biologics that do not behave like antibodies. Identification and characterization of helminth molecules and vesicles and the molecular pathways they target in the host present a unique opportunity to develop tailored drugs inspired by nature that are efficacious, safe, and have minimal immunogenicity. Even so, much work remains to mine and assess this out-of-the-box therapeutic modality. Industry-based organizations need to consider long-haul investments aimed at unraveling and exploiting unique and differentiated mechanisms of action as opposed to toe-dipping entries with an eye on rapid and profitable turnarounds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

