Toxoplasma gondii requires its plant-like heme biosynthesis pathway for infection
- PMID: 32407406
- PMCID: PMC7252677
- DOI: 10.1371/journal.ppat.1008499
Toxoplasma gondii requires its plant-like heme biosynthesis pathway for infection
Abstract
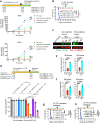
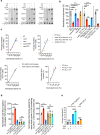
Heme, an iron-containing organic ring, is essential for virtually all living organisms by serving as a prosthetic group in proteins that function in diverse cellular activities ranging from diatomic gas transport and sensing, to mitochondrial respiration, to detoxification. Cellular heme levels in microbial pathogens can be a composite of endogenous de novo synthesis or exogenous uptake of heme or heme synthesis intermediates. Intracellular pathogenic microbes switch routes for heme supply when heme availability fluctuates in their replicative environment throughout infection. Here, we show that Toxoplasma gondii, an obligate intracellular human pathogen, encodes a functional heme biosynthesis pathway. A chloroplast-derived organelle, termed apicoplast, is involved in heme production. Genetic and chemical manipulation revealed that de novo heme production is essential for T. gondii intracellular growth and pathogenesis. Surprisingly, the herbicide oxadiazon significantly impaired Toxoplasma growth, consistent with phylogenetic analyses that show T. gondii protoporphyrinogen oxidase is more closely related to plants than mammals. This inhibition can be enhanced by 15- to 25-fold with two oxadiazon derivatives, lending therapeutic proof that Toxoplasma heme biosynthesis is a druggable target. As T. gondii has been used to model other apicomplexan parasites, our study underscores the utility of targeting heme biosynthesis in other pathogenic apicomplexans, such as Plasmodium spp., Cystoisospora, Eimeria, Neospora, and Sarcocystis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Wu B. Heme biosynthetic pathway in apicomplexan parasites. PhD Dissertation, University of Pennsylvania. 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

