Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis
- PMID: 32407459
- PMCID: PMC7239203
- DOI: 10.1093/cid/ciaa576
Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis
Abstract
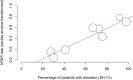
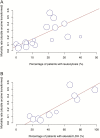
The coronavirus disease 2019 (COVID-19) pandemic spread globally in the beginning of 2020. At present, predictors of severe disease and the efficacy of different treatments are not well understood. We conducted a systematic review and meta-analysis of all published studies up to 15 March 2020, which reported COVID-19 clinical features and/or treatment outcomes. Forty-five studies reporting 4203 patients were included. Pooled rates of intensive care unit (ICU) admission, mortality, and acute respiratory distress syndrome (ARDS) were 10.9%, 4.3%, and 18.4%, respectively. On meta-regression, ICU admission was predicted by increased leukocyte count (P < .0001), alanine aminotransferase (P = .024), and aspartate transaminase (P = .0040); elevated lactate dehydrogenase (LDH) (P < .0001); and increased procalcitonin (P < .0001). ARDS was predicted by elevated LDH (P < .0001), while mortality was predicted by increased leukocyte count (P = .0005) and elevated LDH (P < .0001). Treatment with lopinavir-ritonavir showed no significant benefit in mortality and ARDS rates. Corticosteroids were associated with a higher rate of ARDS (P = .0003).
Keywords: COVID-19; coronavirus; risk factor; severe; treatment.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Worldometers.info. Dover, DE, ; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

